Sedation Effects Produced by a Ciprofol Initial Infusion or Bolus Dose Followed by Continuous Maintenance Infusion in Healthy Subjects: A Phase 1 Trial
- PMID: 34559359
- PMCID: PMC8523013
- DOI: 10.1007/s12325-021-01914-4
Sedation Effects Produced by a Ciprofol Initial Infusion or Bolus Dose Followed by Continuous Maintenance Infusion in Healthy Subjects: A Phase 1 Trial
Abstract
Introduction: The effects of continuous infusions of ciprofol on its pharmacodynamic and pharmacokinetic properties and safety profiles in healthy Chinese subjects were evaluated.
Methods: In this open-label, randomized, two-way cross-over study, subjects received initial doses of continuous ciprofol/propofol as an infusion for 30 min in part 1 (n = 8) and a bolus dose in part 2 (n = 8) followed by maintenance infusions for a total of 4 h in part 1 and 12 h in part 2. Each subject participated in both parts with a washout time of at least 40 h.
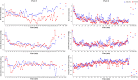
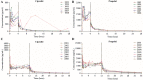
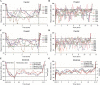
Results: The safety and tolerability parameters of ciprofol were similar to those of propofol, and all treatment-emergent adverse events were mild. The incidences of injection pain and respiratory depression in subjects given ciprofol were lower than those receiving propofol. The pharmacokinetic parameters Cmax, tmax, t1/2, λz and MRT for ciprofol and propofol were similar, while CL, Vd and Vss were statistically significantly different. Pharmacodynamic parameters including the Richmond Agitation Sedation Scale and bispectral index profiles of ciprofol were similar to those of propofol.
Conclusion: Ciprofol has potential for clinical application for continuous intravenous infusion to maintain sedation for 12 h with the same safety, tolerability and efficacy as propofol.
Keywords: Ciprofol; Pharmacodynamics; Pharmacokinetics; Propofol; Safety; Sedation.
© 2021. The Author(s).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

