Treatment options for progression or recurrence of glioblastoma: a network meta-analysis
- PMID: 34559423
- PMCID: PMC8121043
- DOI: 10.1002/14651858.CD013579.pub2
Treatment options for progression or recurrence of glioblastoma: a network meta-analysis
Abstract
Background: Glioblastoma (GBM) is a highly malignant brain tumour that almost inevitably progresses or recurs after first line standard of care. There is no consensus regarding the best treatment/s to offer people upon disease progression or recurrence. For the purposes of this review, progression and recurrence are considered as one entity.
Objectives: To evaluate the effectiveness of further treatment/s for first and subsequent progression or recurrence of glioblastoma (GBM) among people who have received the standard of care (Stupp protocol) for primary treatment of the disease; and to prepare a brief economic commentary on the available evidence.
Search methods: We searched MEDLINE and Embase electronic databases from 2005 to December 2019 and the Cochrane Central Register of Controlled Trials (CENTRAL, in the Cochrane Library; Issue 12, 2019). Economic searches included the National Health Service Economic Evaluation Database (NHS EED) up to 2015 (database closure) and MEDLINE and Embase from 2015 to December 2019.
Selection criteria: Randomised controlled trials (RCTs) and comparative non-randomised studies (NRSs) evaluating effectiveness of treatments for progressive/recurrent GBM. Eligible studies included people with progressive or recurrent GBM who had received first line radiotherapy with concomitant and adjuvant temozolomide (TMZ).
Data collection and analysis: Two review authors independently selected studies and extracted data to a pre-designed data extraction form. We conducted network meta-analyses (NMA) and ranked treatments according to effectiveness for each outcome using the random-effects model and Stata software (version 15). We rated the certainty of evidence using the GRADE approach.
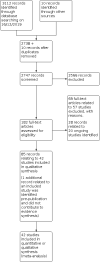
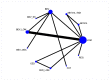
Main results: We included 42 studies: these comprised 34 randomised controlled trials (RCTs) and 8 non-randomised studies (NRSs) involving 5236 participants. We judged most RCTs to be at a low risk of bias and NRSs at high risk of bias. Interventions included chemotherapy, re-operation, re-irradiation and novel therapies either used alone or in combination. For first recurrence, we included 11 interventions in the network meta-analysis (NMA) for overall survival (OS), and eight in the NMA for progression-free survival (PFS). Lomustine (LOM; also known as CCNU) was the most common comparator and was used as the reference treatment. No studies in the NMA evaluated surgery, re-irradiation, PCV (procarbazine, lomustine, vincristine), TMZ re-challenge or best supportive care. We could not perform NMA for second or later recurrence due to insufficient data. Quality-of-life data were sparse. First recurrence (NMA findings) Median OS across included studies in the NMA ranged from 5.5 to 12.6 months and median progression-free survival (PFS) ranged from 1.5 months to 4.2 months. We found no high-certainty evidence that any treatments tested were better than lomustine. These treatments included the following. Bevacizumab plus lomustine: Evidence suggested probably little or no difference in OS between bevacizumab (BEV) combined with lomustine (LOM) and LOM monotherapy (hazard ratio (HR) 0.91, 0.75 to 1.10; moderate-certainty evidence), although BEV + LOM may improve PFS (HR 0.57, 95% confidence interval (CI) 0.44 to 0.74; low-certainty evidence). Bevacizumab monotherapy: Low-certainty evidence suggested there may be little or no difference in OS (HR 1.22, 95% CI 0.84 to 1.76) and PFS (HR 0.90, 95% CI 0.58 to 1.38; low-certainty evidence) between BEV and LOM monotherapies; more evidence on BEV is needed. Regorafenib (REG): REG may improve OS compared with LOM (HR 0.50, 95% CI 0.33 to 0.76; low-certainty evidence). Evidence on PFS was very low certainty and more evidence on REG is needed. Temozolomide (TMZ) plus Depatux-M (ABT414): For OS, low-certainty evidence suggested that TMZ plus ABT414 may be more effective than LOM (HR 0.66, 95% CI 0.47 to 0.92) and may be more effective than BEV (HR 0.54, 95% CI 0.33 to 0.89; low-certainty evidence). This may be due to the TMZ component only and more evidence is needed. Fotemustine (FOM): FOM and LOM may have similar effects on OS (HR 0.89, 95% CI 0.51 to 1.57, low-certainty evidence). Bevacizumab and irinotecan (IRI): Evidence on BEV + irinotecan (IRI) versus LOM for both OS and PFS is very uncertain and there is probably little or no difference between BEV + IRI versus BEV monotherapy (OS: HR 0.95, 95% CI 0.70 to 1.30; moderate-certainty evidence). When treatments were ranked for OS, FOM ranked first, BEV + LOM second, LOM third, BEV + IRI fourth, and BEV fifth. Ranking does not take into account the certainty of the evidence, which also suggests there may be little or no difference between FOM and LOM. Other treatments Three studies evaluated re-operation versus no re-operation, with or without re-irradiation and chemotherapy, and these suggested possible survival advantages with re-operation within the context of being able to select suitable candidates for re-operation. A cannabinoid treatment in the early stages of evaluation, in combination with TMZ, merits further evaluation. Second or later recurrence Limited evidence from three heterogeneous studies suggested that radiotherapy with or without BEV may have a beneficial effect on survival but more evidence is needed. Evidence was insufficient to draw conclusions about the best radiotherapy dosage. Other evidence suggested that there may be little difference in survival with tumour-treating fields compared with physician's best choice of treatment. We found no reliable evidence on best supportive care. Severe adverse events (SAEs) The BEV+LOM combination was associated with significantly greater risk of SAEs than LOM monotherapy (RR 2.51, 95% CI 1.72 to 3.66, high-certainty evidence), and ranked joint worst with cediranib + LOM (RR 2.51, 95% CI 1.29 to 4.90; high-certainty evidence). LOM ranked best and REG ranked second best. Adding novel treatments to BEV was generally associated with a higher risk of severe adverse events compared with BEV alone.
Authors' conclusions: For treatment of first recurrence of GBM, among people previously treated with surgery and standard chemoradiotherapy, the combination treatments evaluated did not improve overall survival compared with LOM monotherapy and were often associated with a higher risk of severe adverse events. Limited evidence suggested that re-operation with or without re-irradiation and chemotherapy may be suitable for selected candidates. Evidence on second recurrence is sparse. Re-irradiation with or without bevacizumab may be of value in selected individuals, but more evidence is needed.
Antecedentes: El glioblastoma (GBM) es un tumor cerebral altamente maligno que casi inevitablemente progresa o recidiva después de un tratamiento de primera línea. No hay consenso sobre el mejor o los mejores tratamientos que se pueden ofrecer a las personas que presentan progresión o recidiva de la enfermedad. A los efectos de la presente revisión, la progresión y la recidiva se consideran como una sola entidad.
Objetivos: Evaluar la efectividad de los tratamientos adicionales para la primera y subsiguiente progresión o recidiva del glioblastoma (GBM) entre las personas que han recibido atención estándar (protocolo Stupp) para el tratamiento primario de la enfermedad, así como preparar un breve comentario económico sobre la evidencia disponible. MÉTODOS DE BÚSQUEDA: Se realizaron búsquedas en las bases de datos electrónicas de MEDLINE y Embase desde 2005 hasta diciembre de 2019 y en el Registro Cochrane central de ensayos controlados (Cochrane Central Register of Controlled Trials) (CENTRAL, en la Cochrane Library; Número 12, 2019). Las búsquedas económicas incluyeron la National Health Service Economic Evaluation Database (NHS EED) hasta 2015 (cierre de la base de datos) y MEDLINE y Embase desde 2015 hasta diciembre de 2019. CRITERIOS DE SELECCIÓN: Ensayos controlados aleatorizados (ECA) y estudios comparativos no aleatorizados (no ECA) que evaluaron la efectividad de los tratamientos para el GBM progresivo/recidivante. Los estudios elegibles incluyeron personas con GBM progresivo o recidivante que habían recibido radioterapia de primera línea con temozolomida (TMZ) concomitante y adyuvante. OBTENCIÓN Y ANÁLISIS DE LOS DATOS: Dos autores de la revisión de forma independiente seleccionaron los estudios y extrajeron los datos en un formulario de extracción de datos prediseñado. Se realizaron metanálisis en red (MAR) y los tratamientos se clasificaron según la efectividad de cada desenlace, mediante el modelo de efectos aleatorios y el software Stata (versión 15). La certeza de la evidencia se evaluó mediante los criterios GRADE.
Resultados principales: Se incluyeron 42 estudios, que comprendieron 34 ensayos controlados aleatorizados (ECA) y ocho estudios no aleatorizados (no ECA), con 5236 participantes. Se consideró que la mayoría de los ECA tuvieron bajo riesgo de sesgo y que los no ECA tuvieron alto riesgo de sesgo. Las intervenciones incluyeron quimioterapia, reoperación, reirradiación y tratamientos nuevos, ya sea utilizadas solos o en combinación. Para la primera recidiva se incluyeron 11 intervenciones en el metanálisis en red (MAR) para la supervivencia general (SG), y ocho para la supervivencia sin progresión (SSP). La lomustina (LOM; también conocida como CCNU) fue el comparador más frecuente y se utilizó como tratamiento de referencia. Ningún estudio en el MAR evaluó la cirugía, la reirradiación, la PCV (procarbazina, lomustina, vincristina), la reexposición a TMZ o el mejor tratamiento de apoyo. No fue posible realizar un MAR para una segunda o posterior recidiva debido a que los datos no fueron suficientes. Los datos de calidad de vida fueron escasos. Primera recidiva (hallazgos del MAR) La mediana de la SG en los estudios incluidos en el MAR varió entre 5,5 y 12,6 meses y la mediana de la supervivencia sin progresión (SSP) varió entre 1,5 y 4,2 meses. No se encontró evidencia de certeza alta de que los tratamientos probados fueran mejores que la lomustina. Estos tratamientos incluyeron los siguientes. Bevacizumab más lomustina: La evidencia indicó probablemente poca o ninguna diferencia en la SG entre el bevacizumab (BEV) combinado con lomustina (LOM) y la monoterapia con LOM (cociente de riesgos instantáneo [CRI] 0,91; 0,75 a 1,10; evidencia de certeza moderada), aunque BEV + LOM puede mejorar la SSP (CRI 0,57; intervalo de confianza [IC] del 95%: 0,44 a 0,74; evidencia de certeza baja). Monoterapia con bevacizumab: La evidencia de certeza baja indicó que puede haber poca o ninguna diferencia en la SG (CRI 1,22; IC del 95%: 0,84 a 1,76) y la SSP (CRI 0,90; IC del 95%: 0,58 a 1,38; evidencia de certeza baja) entre las monoterapias con BEV y LOM; se necesita más evidencia sobre el BEV. Regorafenib (REG): El REG puede mejorar la SG en comparación con la LOM (CRI 0,50; IC del 95%: 0,33 a 0,76; evidencia de certeza baja). La evidencia sobre la SSP fue de certeza muy baja y se necesita más evidencia sobre el REG. Temozolomida (TMZ) más Depatux‐M (ABT414): En cuanto a la SG, evidencia de certeza baja indicó que TMZ más ABT414 puede ser más efectiva que LOM (CRI 0,66; IC del 95%: 0,47 a 0,92) y puede ser más efectiva que BEV (CRI 0,54; IC del 95%: 0,33 a 0,89; evidencia de certeza baja). Lo anterior se puede deber solamente al componente de TMZ, y se necesita más evidencia. Fotemustina (FOM): FOM y LOM pueden tener efectos similares sobre la SG (CRI 0,89; IC del 95%: 0,51 a 1,57, evidencia de certeza baja). Bevacizumab e irinotecan (IRI): La evidencia sobre BEV + irinotecan (IRI) versus LOM para la SG y la SSP no está clara y probablemente hay poca o ninguna diferencia entre BEV + IRI versus la monoterapia con BEV (SG: CRI 0,95; IC del 95%: 0,70 a 1,30; evidencia de certeza moderada). Cuando los tratamientos se clasificaron según la SG, FOM se clasificó primero, BEV + LOM segundo, LOM tercero, BEV + IRI cuarto, y BEV quinto. La clasificación no tiene en cuenta la certeza de la evidencia, lo que también indica que puede haber poca o ninguna diferencia entre FOM y LOM. Otros tratamientos Tres estudios evaluaron la reoperación versus ninguna reoperación, con o sin reirradiación y quimioterapia, e indicaron posibles ventajas en la supervivencia con la reoperación, en el contexto de poder seleccionar candidatos adecuados para esta intervención. Un tratamiento con cannabinoides en las primeras etapas de evaluación, en combinación con TMZ, merece evaluación adicional. Segunda o posterior recidiva La evidencia limitada de tres estudios heterogéneos indicó que la radioterapia con o sin BEV puede tener un efecto beneficioso sobre la supervivencia, pero se necesita más evidencia. La evidencia no fue suficiente para establecer conclusiones sobre la mejor dosis de radioterapia. Otra evidencia indicó que puede haber poca diferencia en la supervivencia con los campos de tratamiento del tumor en comparación con la mejor opción de tratamiento del médico. No se encontró evidencia fiable sobre el mejor tratamiento de apoyo. Eventos adversos graves (EAG) La combinación BEV + LOM se asoció con un riesgo significativamente mayor de EAG que la monoterapia con LOM (RR 2,51; IC del 95%: 1,72 a 3,66; evidencia de certeza alta), y se clasificó peor junto con cediranib + LOM (RR 2,51; IC del 95%: 1,29 a 4,90; evidencia de certeza alta). LOM se clasificó como el mejor y REG como el segundo mejor. Agregar nuevos tratamientos al BEV se asoció generalmente con un mayor riesgo de eventos adversos graves, en comparación con BEV solo.
Conclusiones de los autores: Para el tratamiento de la primera recidiva del GBM en personas tratadas previamente con cirugía y quimiorradioterapia estándar, los tratamientos combinados evaluados no mejoraron la supervivencia general en comparación con la monoterapia con LOM, y a menudo se asociaron con un mayor riesgo de eventos adversos graves. Hay evidencia limitada que indica que la reoperación con o sin reirradiación y quimioterapia puede ser adecuada para candidatos seleccionados. La evidencia sobre la segunda recidiva es escasa. La reirradiación con o sin bevacizumab puede ser de valor en determinados individuos, pero se necesita más evidencia.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Catherine McBain: none declared Theresa Lawrie: none declared Ewelina Rogozinska: none declared Ashleigh Kernohan: none declared Tomos Robinson: none declared Sarah Jefferies: none declared
Figures






Update of
References
References to studies included in this review
Azoulay 2017 {published data only}
-
- Azoulay M, Santos F, Shenouda G, Petrecca K, Oweida A, Guiot MC, et al. Benefit of re-operation and salvage therapies for recurrent glioblastoma multiforme: results from a single institution. Journal of Neuro-oncology 2017;132(3):419-26. - PubMed
Batchelor 2013 {published data only}
-
- Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. Journal of Clinical Oncology 2013;31(26):3212-8. - PMC - PubMed
Bloch 2017 {published data only}
-
- Bloch O, Shi Q, Anderson SK, Knopp M, Raizer J, Clarke J, et al. Alliance a071101: a phase II randomized trial comparing the efficacy of heat shock protein peptide complex-96 (HSPPC-96) vaccine given with bevacizumab versus bevacizumab alone in the treatment of surgically resectable recurrent glioblastoma. Neuro-oncology 2017;19(suppl 6):vi29. [DOI: 10.1093/neuonc/nox168.110] - DOI
Brandes 2016a {published data only}
-
- Brandes AA, Carpentier AF, Kesari S, Sepulveda-Sanchez JM, Wheeler HR, Chinot O, et al. A Phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro-oncology 2016;18(8):1146-56. - PMC - PubMed
Brandes 2016b {published data only}
Brandes 2018 {published data only}
Brown 2016 {published data only}
Cloughesy 2017 {published data only}
-
- Cloughesy T, Finocchiaro G, Belda-Iniesta C, Recht L, Brandes AA, Pineda E, et al. Randomized, double-blind, placebo-controlled, multicenter phase II study of onartuzumab plus bevacizumab versus placebo plus bevacizumab in patients with recurrent glioblastoma: efficacy, safety, and hepatocyte growth factor and O6-methylguanine-DNA methyltransferase biomarker analyses. Journal of Clinical Oncology 2017;35(3):343-51. - PubMed
Cloughesy 2018 {published data only}
-
- Cloughesy T, Brenner AJ, Butowski N, Cohen YC, Lowenton-Spier N, Wen P. Results of the globe study: a phase 3, randomized, controlled, double-arm, open label, multi-center study of VB-111 combined with bevacizumab vs. bevacizumab monotherapy in patients with recurrent glioblastoma. Neuro-oncology 2018;20(suppl_6):vi4-5. - PMC - PubMed
-
- Cloughesy TF, Brenner A, Groot JF, Butowski NA, Zach L, Campian Jl, et al. A randomized controlled phase III study of VB-111 combined with bevacizumab vs. bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE). Neuro-oncology 2020;22(5):705-17. [DOI: 10.1093/neuonc/noz232] - DOI - PMC - PubMed
-
- NCT02511405. A phase 3, pivotal trial of VB-111 plus bevacizumab vs. bevacizumab in patients with recurrent glioblastoma (GLOBE) [A phase 3, randomized, controlled, double-arm, open-label, multi-center study of VB-111 combined with bevacizumab vs. bevacizumab monotherapy in patients with recurrent glioblastoma]. www.clinicaltrials.gov/ct2/show/NCT02511405 (first received 30 July 2015).
Cuncannon 2019 {published data only}
Dresemann 2010 {published data only}
-
- Dresemann G, Weller M, Rosenthal MA, Wedding U, Wagner W, Engel E, et al. Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. Journal of Neuro-oncology 2010;96(3):393-402. - PubMed
Duerinck 2018 {published data only}
-
- Duerinck J, Du Four S, Bouttens F, Andre C, Verschaeve V, Van Fraeyenhove F, et al. Randomized phase II trial comparing axitinib with the combination of axitinib and lomustine in patients with recurrent glioblastoma. Journal of Neuro-oncology 2018;136(1):115-25. - PubMed
-
- Duerinck J, Du Four S, Bouttens F, Verschaeve V, Andre C, Van Fraeyenhove F, et al. Axitinib for the treatment of patients with recurrent glioblastoma, final results from a randomized phase II clinical trial. Neuro-oncology 2017;18(suppl_4):iv41-42. [DOI: 10.1093/neuronc/now188.142] [PMID: ] - DOI
-
- Duerinck J, Du Four S, Bouttens F, Verschaeve V, Andre C, Van Fraeyenhove F, et al. Randomized phase II study of axitinib alone or combined with lomustine in patients with recurrent glioblastoma. Journal of Clinical Oncology 2016;34(15 suppl):2038.
-
- Duerinck J, Du Four S, Vandervorst F, D'Haene N, Mercier M, Michotte A, et al. Randomized phase II study of axitinib versus physicians best alternative choice of therapy in patients with recurrent glioblastoma. Journal of Neuro-oncology 2016;128:147-55. - PubMed
Field 2015 {published data only}
-
- Field KM, King MT, Simes J, Espinoza D, Barnes EH, Sawkins K, et al. Health-related quality of life outcomes from CABARET: a randomized phase 2 trial of carboplatin and bevacizumab in recurrent glioblastoma. Journal of Neuro-oncology 2017;133(3):623-31. - PubMed
-
- Hovey EJ, Field KM, Rosenthal M, Nowak AK, Cher L, Wheeler H, et al. Continuing or ceasing bevacizumab at disease progression: results from the CABARET study, a prospective randomized phase II trial in patients with recurrent glioblastoma. Journal of Clinical Oncology 2015;33(15_suppl):2003. [DOI: 10.1200/jco.2015.33.15_suppl.2003] - DOI
Friedman 2009 {published data only}
-
- Anonymous. Erratum: a phase II, randomized, non-comparative clinical trial of the effect of bevacizumab (BV) alone or in combination with irinotecan (CPT) on 6-month progression free survival (PFS6) in recurrent, treatment-refractory glioblastoma (GBM) (Journal of Clinical Oncology (2008) 26 (15S)). Journal of Clinical Oncology 2011;29(10):1394.
-
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. Journal of Clinical Oncology 2009;27(28):4733-40. - PubMed
Galanis 2017 {published data only}
-
- Galanis E, Anderson SK, Butowski NA, Hormigo A, Schiff D, Tran DD, et al. NCCTG N1174: phase I/comparative randomized phase (Ph) II trial of TRC105 plus bevacizumab versus bevacizumab in recurrent glioblastoma (GBM) (Alliance). In: Journal of Clinical Oncology. Vol. 35. 2017:2023.
-
- NCT01648348. Bevacizumab with or without anti-endoglin monoclonal antibody TRC105 in treating patients with recurrent glioblastoma multiforme. clinicaltrials.gov/ct2/show/results/NCT01648348 (first received 20 July 2012).
Galanis 2019 {published data only}
-
- Galanis E, Anderson SK, Anastasiadis P, Tran DD, Jeyapalan SA, Anderson DM, et al. NCCTG N0872 (Alliance): a randomized placebo-controlled phase II trial of bevacizumab plus dasatinib in patients with recurrent glioblastoma (GBM). Journal of Clinical Oncology 2015;33(15_suppl 1):2004. [DOI: 10.1200/jco.2015.33.15_suppl.2004] - DOI
Gilbert 2017 {published data only}
Heiland 2016 {published data only}
-
- Heiland DH, Masalha W, Franco P, Machein MR, Weyerbrock A. Progression-free and overall survival in patients with recurrent glioblastoma multiforme treated with last-line bevacizumab versus bevacizumab/lomustine. Journal of Neuro-oncology 2016;126(3):567-75. - PubMed
Kim 2015 {published data only}
-
- Kim HR, Kim KH, Kong DS, Seol HJ, Nam DH, Lim DH, et al. Outcome of salvage treatment for recurrent glioblastoma. Journal of Clinical Neuroscience 2015;22(3):468-73. - PubMed
Kunwar 2010 {published data only}
Lombardi 2019 {published data only}
-
- EUCTR2014-003722-41-IT. Clinical trial to evaluate the regorafenib therapy in patients with brain tumor progression after standard therapy (Radiotherapy and Temozolomide) with or without bevacizumab. www.clinicaltrialsregister.eu/ctr-search/trial/2014-003722-41/IT (first received 29 January 2015).
-
- Lombardi G, De Salvo G, Ruda R, Franceschi E, Eoli M, Faedi M, et al. Updated results of REGOMA: a randomized, multicenter, controlled open-label Phase II clinical trial evaluating regorafenib in relapsed glioblastoma patients. Journal of Clinical Oncology 2018;36(15_suppl):2047.
-
- Lombardi G, De Salvo GL, Brandes AA, Eoli M, Ruda R, Faedi M, et al. REGOMA: a randomized, multicenter, controlled open-label phase II clinical trial evaluating regorafenib activity in relapsed glioblastoma patients. Journal of Clinical Oncology 2017;15_suppl:v610. [DOI: 10.1200/JCO.2017.35.15_suppl.TPS2085] - DOI
-
- Lombardi G, De Salvo GL, Brandes AA, Eoli M, Ruda R, Faedi M, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncology 2019;20(1):110-9. - PubMed
-
- Lombardi G, De Salvo GL, Ruda R, Franceschi E, Eoli M, Faedi M, et al. Updated results of REGOMA: A randomized, multicenter, controlled open-label phase II clinical trial evaluating regorafenib in relapsed glioblastoma (GBM) patients (PTS). Journal of Clinical Oncology 2018;36(15_suppl):2047. [DOI: 10.1200/JCO.2018.36.15_suppl.2047] - DOI
Modh 2018 {published data only}
-
- Modh A, Bergman D, Hanna R, Schultz L, Snyder J, Mikkelsen T, et al. Randomized prospective trial of stereotactic radiosurgery versus chemotherapy for recurrent malignant glioma after second-line chemotherapy. Neuro-oncology 2019;Conference: ARS 101st Annual Meeting. United States.103(5 Supplement):E17-8.
-
- Modh A, Bergman D, Schultz L, Snyder J, Mikkelsen T, Ryu S, et al. Randomized prospective trial of stereotactic radiosurgery versus chemotherapy for recurrent malignant glioma after second-line chemotherapy. Neuro-oncology. Conference abstract. 23rd Annual Scientific Meeting and Education Day of the Society for Neuro-Oncology 2018;20(Supplement 6):vi226.
-
- Walbert T, Modh A, Bergman D, Schultz L, Snyder J, Mikkelsen T, et al. Randomized prospective trial of stereotactic radiosurgery versus chemotherapy for recurrent malignant glioma after second-line chemotherapy. Neuro-oncology 2019;Conference: 71st Annual Meeting of the American Academy of Neurology, AAN 2019. United States. 92(15 Supplement 1).
Narita 2019 {published data only}
-
- Arakawa Y, Nagane M, Hirose Y, Sasada T, Yamada A, Itoh K, et al. Randomized, double-blind, phase III trial of a personalized peptide vaccination for recurrent glioblastoma patients. Cancer Science 2018;109:1126.
-
- Fujimaki T, Itoh K, Terasaki M, Narita Y, Arakawa Y, Sugiyama K, et al. Trials of a personalized peptide vaccine (ITK-1) for patients with recurrent or progressive glioblastoma (GBM). In: Neuro-oncology. Vol. 19. 2017:iii52.
Omuro 2018 {published data only}
-
- Reardon DA, Omuro A, Brandes AA, Rieger J, Wick A, Sepulveda J, et al. Randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. In: Neuro-oncology. Conference: 5th Quadrennial Meeting of the World Federation of Neuro-oncology Societies, WFNOS. Switzerland. Vol. 19. 2017:iii21.
-
- Sampson JH, Vlahovic G, Sahebjam S, Omuro AMP, Baehring JM, Hafler DA, et al. Preliminary safety and activity of nivolumab and its combination with ipilimumab in recurrent glioblastoma (GBM): CheckMate 143. In: Journal of Clinical Oncology. Conference abstract. Vol. 33. 2015:3010.
Puduvalli 2018 {published data only}
-
- Phase I/II adaptive randomized trial of bevacizumab versus bevacizumab plus vorinostat in adults with recurrent glioblastoma. ClinicalTrials.gov 2018:https://clinicaltrials.gov/ct2/show/results/NCT01266031.
-
- Puduvalli V, Yuan Y, Armstrong T, Wu J, Giglio P, Xu J, et al. A bayesian adaptive randomized phase ii trial of bevacizumab versus bevacizumab plus vorinostat in adults with recurrent glioblastoma final results, Conference Abstract: 23rd Annual Scientific Meeting and Education Day of the Society for Neuro-oncology. United States. In: Neuro-oncology. Vol. 20. 2018:vi13.
-
- Puduvalli VK, Wu J, Yuan Y, Armstrong TS, Groves MD, Raizer JJ, et al. Brain tumor trials collaborative bayesian adaptive randomized phase II trial of bevacizumab plus vorinostat versus bevacizumab alone in adults with recurrent glioblastoma (BTTC-1102). In: Journal of Clinical Oncology. Vol. 33. 2015.
Reardon 2011 {published data only}
Reardon 2015b {published data only}
Reardon 2018a {published data only}
-
- Reardon DA, Lassman AB, Schiff D, Yunus SA, Gerstner ER, Cloughesy TF, et al. Phase 2 and biomarker study of trebananib, an angiopoietin-blocking peptibody, with and without bevacizumab for patients with recurrent glioblastoma. Cancer 2018;124(11):1438-48. - PubMed
Reardon 2018b {published data only}
-
- NCT02337491. Pembrolizumab +/- Bevacizumab for Recurrent GBM. ClinicalTrials.gov 2015.
-
- Reardon DA, Nayak L, Peters KB, Clarke JL, Jordan JT, De Groot JF, et al. Phase II study of pembrolizumab or pembrolizumab plus bevacizumab for recurrent glioblastoma (rGBM) patients. In: Journal of Clinical Oncology. Conference abstract. Vol. 36. 2018:2006.
Reardon 2020 {published data only}
-
- Reardon DA, Desjardins A, Vredenburgh JJ, O'Rouke DM, Tran DD, Fink KL. Rindopepimut with bevacizumab for patients with relapsed EGFRvIII-expressing glioblastoma (ReACT): results of a double-blind randomized phase II trial. Clinical Cancer Research 2020;26:1586-94. - PubMed
-
- Reardon DA, Schuster J, Tran DD, Fink KL, Nabors LB, Li G, et al. ReACT: overall survival from a randomized phase II study of rindopepimut (CDX-110) plus bevacizumab in relapsed glioblastoma. Journal of Clinical Oncology 2015;33(15):SUPPL. 1.
-
- Reardon DA, Schuster JM, Tran DD, Fink KL, Nabors LB, Li G, et al. React: overall survival from a randomized phase II study of rindopepimut (CDX-110) plus bevacizumab in relapsed glioblastoma. In: Clinical Neurosurgery. Vol. 62. 2015:198-9.
-
- Sampson JH, Desjardins A, Schuster J, Tran D, Fink K, Nabors L, et al. ReACT: a randomized phase II study of rindopepimut (CDX-110) plus bevacizumab in relapsed glioblastoma national brain tumor society mahaley award. In: Journal of Neurosurgery. Vol. 124. 2016:A1152.
Santos 2018 {published data only}
-
- RBR-3byz2c. Evaluation of inflammatory substances in the blood of patients with recurrent glioblastoma in treatment with inhalation of perillyl alcohol associated with reduced carbohydrate diet and supplementation with omega 3. ICTRP Search Portal. World Health Organization 2018:RBR-3byz2c.
-
- RBR-8x8fd9. Low sugar diet combined with nasal administration of perillyl alcohol: strategy therapy for resistant glioblastoma multiforme to chemotherapy and radiotherapy. ICTRP Search Portal. World Health Organization 2016.
Scorsetti 2015 {published data only}
Stupp 2012 {published data only}
-
- Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, et al. NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. European Journal of Cancer 2012;48(14):2192-202. - PubMed
Suchorska 2016 {published data only}
Taal 2014 {published data only}
-
- Beije N, Kraan J, Taal W, Holt B, Oosterkamp HM, Walenkamp AM, et al. Prognostic value and kinetics of circulating endothelial cells in patients with recurrent glioblastoma randomised to bevacizumab plus lomustine, bevacizumab single agent or lomustine single agent. A report from the Dutch Neuro-oncology Group BELOB trial. British Journal of Cancer 2015;113(2):226-31. - PMC - PubMed
-
- Dirven L, den Bent MJ, Bottomley A, Meer N, Holt B, Vos MJ, et al. The impact of bevacizumab on health-related quality of life in patients treated for recurrent glioblastoma: results of the randomised controlled phase 2 BELOB trial. European Journal of Cancer 2015;51(10):1321-30. - PubMed
-
- Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. The Lancet 2014;15(9):943-53. - PubMed
Tsien 2019 {published data only}
-
- Tsien C, Pugh S, Dicker AP, Raizer JJ, Matuszak MM, Lallana E, et al. Randomized phase II trial of re-irradiation and concurrent bevacizumab versus bevacizumab alone as treatment for recurrent glioblastoma (NRG Oncology/RTOG 1205): initial outcomes and RT plan quality report. In: International Journal of Radiation Oncology, Biology, Physics. Conference abstract. Vol. 105. Suppl 1. 2019:S78.
Twelves 2017 {published data only}
-
- Twelves C, Short S, Wright S. A two-part safety and exploratory efficacy randomized double-blind, placebo-controlled study of a 1: 1 ratio of the cannabinoids cannabidiol and delta-9-tetrahydrocannabinol (CBD: tHC) plus dose-intense temozolomide in patients with recurrent glioblastoma multiforme (GBM). Journal of Clinical Oncology 2017;35(15):2046.
van den Bent 2018 {published data only}
-
- Clement P, Van Den Bent M, Eoli M, Sepulveda JM, Walenkamp AME, Frenel JS, et al. Health related quality of life and neurological deterioration free survival in intellance 2/EORTC trial 1410, a randomized phase ii study on ABT414 in recurrent EGFR amplified glioblastoma. In: Neuro-oncology. Vol. Conference: 22nd Annual Scientific Meeting and Education Day of the Society for Neuro-oncology. United States.19. 2017:vi211.
-
- EUCTR2014-004438-24-GB. ABT-414 alone or ABT-414 plus temozolomide versus temozolomide or lomustine alone in subjects with recurrent glioblastoma. 2015. EU Clinical Trial Register.
-
- Van Den Bent M, Eoli M, Sepulveda JM, Smits M, Walenkamp AME, Frenel JS, et al. First results of the randomized phase ii study on depatux-m alone, depatux-m in combination with temozolomide and either temozolomide or lomustine in recurrent EGFR amplified glioblastoma: first report from intellance 2/eortc trial 1410. Neuro-oncology 2017;Conference: 22nd Annual Scientific Meeting and Education Day of the Society for Neuro-Oncology. United States.19(Supplement 6):vi316.
-
- Van Den Bent M, French P, Eoli M, Sepulvado J, Walenkamp AME, Weller M, et al. Updated results of the intellance 2/EORTC trial 1410 randomized phase II study on depatux-m alone, depatux-m in combination with temozolomide (TMZ) and either TMZ or lomustine (LOM) in recurrent egfr amplified glioblastoma (NCT02343406). Neuro-oncology 2018;20:iii240-1.
-
- Van Den Bent M, French P, Eoli M, Sepulveda J, Walenkamp AME, Frenel JS, et al. Two-year results of the intellance 2/eortc trial 1410 randomized phase ii study on depatux-m alone, depatux-m combined with temozolomide (TMZ) and either tmz or lomustine in recurrent EGFR amplified glioblastoma (NCT02343406). Neuro-oncology 2018;Conference: 23rd Annual Scientific Meeting and Education Day of the Society for Neuro-Oncology. United States. 20(Supplement 6):vi20.
Weathers 2016 {published data only}
-
- Weathers SP, Han X, Liu DD, Conrad CA, Gilbert MR, Loghin ME, et al. A randomized phase II trial of standard dose bevacizumab versus low dose bevacizumab plus lomustine (CCNU) in adults with recurrent glioblastoma. In: Journal of Clinical Oncology. Conference abstract. Vol. 33. 15 SUPPL. 1. 2015:2005. - PMC - PubMed
Wick 2010 {published data only}
Wick 2014 {published data only}
-
- Ficke H, Hanke N, Kunz C, Wick W, Lehr T. Aunercept plus radiotherapy in relapsed glioblastoma. Update on five years overall survival of study NCT01071837 and development of a population PK - tumour growth inhibition - survival model. In: Neuro-oncology. 2018:vi3.
-
- Wick W, Fricke H, Junge K, Kobyakov G, Martens T, Heese O, et al. A phase II randomized study of weekly APG101+reirradiation versus reirradiation in progressive glioblastoma. Clinical Cancer Research 2014;20(24):6304-13. - PubMed
Wick 2017 {published data only}
-
- Taphoorn M, Bottomley A, Coens C, Reijneveld J, Gorlia T, Brandes AA, et al. Health-related quality of life (HRQoL) in patients with progressive glioblastoma treated with combined bevacizumab and lomustine versus lomustine only (randomized phase iii EORTC study 26101). In: Neuro-oncology. Conference abstract. Vol. 18. 2016:vi157.
-
- Taphoorn MJB, Bottomley A, Coens C, Reijneveld JC, Gorlia T, Brandes AA, et al. Health-related quality of life (HRQOL) in patients with progressive glioblastoma treated with combined bevacizumab and lomustine versus lomustine only (randomized phase III eortc study 26101). In: Neuro-oncology. Vol. 18. 2017:iv11-2.
-
- Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, et al. Lomustine and bevacizumab in progressive glioblastoma. New England Journal of Medicine 2017;377(20):1954-1963. - PubMed
-
- Wick W, Stupp R, Gorlla T, Bendszus M, Sahm F, Bromberg JE, et al. Phase II part of EORTC study 26101: the sequence of bevacizumab and lomustine in patients with first recurrence of a glioblastoma. In: Journal of Clinical Oncology. Vol. 34. 2016:Suppl 15.
References to studies excluded from this review
Abacioglu 2011 {published data only}
-
- Abacioglu U, Caglar HB, Yumuk PF, Akgun Z, Atasoy BM, Sengoz M. Efficacy of protracted dose-dense temozolomide in patients with recurrent high-grade glioma. Journal of Neuro-oncology 2011;103(3):585-93. - PubMed
Abdel‐Rahman 2015 {published data only}
-
- Abdel-Rahman O, Fouad M. Irinotecan-based regimens for recurrent glioblastoma multiforme: [corrected] a systematic review. Expert Review of Neurotherapeutics 2015;15(11):1255-70. - PubMed
ACTRN12615001072505 2015 {published data only}
-
- ACTRN12615001072505. A phase II randomised placebo-controlled, double blind, multisite study of acetazolamide versus placebo for management of cerebral oedema in recurrent and/or progressive high grade glioma requiring treatment with dexamethasone – The ACED trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=368253 (first received 19 August 2015).
Ahluwalia 2018 {published data only}
-
- Ahluwalia M, Peereboom D, Schilero C, Forst D, Wong E, Wen P, et al. Randomized phase 2 open label study of nivolumab plus standard dose bevacizumab versus nivolumab plus low dose bevacizumab in recurrent glioblastoma. Neuro-oncology 2018;20((suppl_6)):vi234.
-
- NCT03452579. Nivolumab plus standard dose bevacizumab versus nivolumab plus low dose bevacizumab in GBM. clinicaltrials.gov/ct2/show/NCT03452579 (first received 2 March 2018).
Aoki 2016 {published data only}
Bartsch 2005 {published data only}
-
- Bartsch R, Weitmann HD, Pennwieser W, Wenzel C, Muschitz S, Baldass M, et al. Retrospective analysis of re-irradiation in malignant glioma: a single-center experience. Wiener klinische Wochenschrift 2005;117(23-4):821-6. - PubMed
Bogdahn 2011 {published data only}
Boiardi 2008 {published data only}
-
- Boiardi A, Silvani A, Eoli M, Lamperti E, Salmaggi A, Gaviani P, et al. Treatment of recurrent glioblastoma: can local delivery of mitoxantrone improve survival? Journal of Neuro-oncology 2008;88(1):105-13. - PubMed
Brada 2010 {published data only}
-
- Brada M, Stenning S, Gabe R, Thompson LC, Levy D, Rampling R, et al. Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high grade glioma. Journal of Clinical Oncology 2010;28(30):4601-8. - PubMed
Brandes 2009 {published data only}
-
- Brandes AA, Tosoni A, Franceschi E, Blatt V, Santoro A, Faedi M, et al. Fotemustine as second-line treatment for recurrent or progressive glioblastoma after concomitant and/or adjuvant temozolomide: a phase II trial of Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Cancer chemotherapy and pharmacology 2009;64(4):769-75. - PMC - PubMed
Chen 2015 {published data only}
Cher 2017 {published data only}
-
- Cher L, Nowak AK, Iatropoulos G, Lee WS, Lee SY, Shim SR, et al. A multicenter, 3-arm, open-label, phase Ila clinical trial to evaluate the safety and efficacy of tanibirumab (VEGFR2 mAb), in patients with recurrent glioblastoma (GBM). Journal of Clinical Oncology 2017;35:15 Supplement 1 (no pagination).
CTRI/2018/01/011542 {published data only}
-
- CTRI/2018/01/011542. Mebendazole in brain tumor. ClinicalTrials.gov 2018.
Dixit 2015 {published data only}
-
- Dixit S, Hingorani M. Comparative efficacy of bevacizumab and PCV chemotherapy in recurrent glioblastoma. Clinical Oncology 2015;27(4):246-7. - PubMed
Duque 2017 {published data only}
-
- Duque AED, De Feyter H, Kemble G, McCulloch W, Brenner AJ. A phase 2 study to determine the efficacy and safety of TVB-2640 in combination with bevacizumab in patients with first relapse of high grade astrocytoma. Journal of Clinical Oncology 2017;35:15.
Ellingson 2019 {published data only}
-
- Ellingson BM, Raymond C, Yao J, Chakhoyan A, Turley D, Tsung J, et al. Quantitative radiographic analysis of phase II and III trials in recurrent glioblastoma treated with VB111 with or without bevacizumab or bevacizumab monotherapy. Journal of Clinical Oncology 2019;37:No pagination.
Fogh 2010 {published data only}
Gan 2015 {published data only}
-
- Gan HK, Papadopoulos KP, Fichtel L, Lassman AB, Merrell R, Van Den Bent MJ, et al. Phase I study of ABT-414 mono-or combination therapy with temozolomide (TMZ) in recurrent glioblastoma (GBM). Journal of Clinical Oncology 2015;33(15 Supp. 1):2016.
Gatson 2015 {published data only}
Haslund 2016 {published data only}
-
- Haslund C, Muhic A, Lukacova S, Lund B, Lassen-Ramshad Y, Meyer M, et al. An open-labelled, randomized phase II study in patients with recurrent glioblastoma multiforme comparing progression free survival of alecsat (autologous lymphoid effector cells specific against tumour-cells) versus bevacizumab/irinotecan. In: Neuro-oncology. Vol. 18. 2016:iv6.
Hong 2013 {published data only}
-
- Hong B, Wiese B, Bremer M, Heissler HE, Heidenreich F, Krauss JK, et al. Multiple microsurgical resections for repeated recurrence of glioblastoma multiforme. American Journal of Clinical Oncology 2013;36(3):261-8. - PubMed
Idbaih 2016 {published data only}
-
- Idbaih A, Clement PM, Vos FYF, Platten M, Mulholland P, Taphoorn MJB, et al. First results of the randomized phase II tavarec trial on temozolomide with or without bevacizumab in 1P/19Q intact 1st recurrence grade II and III glioma. In: Neuro-oncology. Vol. 18. 2016:iv13. [DOI: 10.1093/neuonc/now188.041] - DOI
Immonen 2004 {published data only}
-
- Immonen A, Vapalahti M, Tyynela K, Hurskainen H, Sandmair A, Vanninen R, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Molecular Therapy 2004;10(5):967-72. - PubMed
Ji 2016 {published data only}
Kaloshi 2015 {published data only}
-
- Kaloshi G, Diamandi P, Cakani B, Brace G, Rroji A, Petrela M. The added value of bevacizumab concomitantly administered with carboplatin versus carboplatin alone in patients with recurrent glioblastomas. Tumori 2015;101(1):41-5. - PubMed
Kesari 2017 {published data only}
-
- Kesari S, Tran D, Read W, Ahluwalia M, Villano J, Toms S, et al. Tumor treating fields with second line treatment compared to second line treatment alone in patients at first recurrence of glioblastoma-a post hoc analysis of the EF-14 phase 3 clinical trial. Conference abstract. Conference: 22nd Annual Scientific Meeting and Education Day of the Society for Neuro-Oncology. United States 2017;19(Supplement 6):vi13.
Kinzel 2018 {published data only}
-
- Kinzel A, Lavy-Shahaf G, Kirson E. Tumor treating fields (TTfields) in combination with lomustine (CCNU) in the EF-14 phase 3 clinical study-a safety analysis. In: Neuro-oncology. Vol. 20. 2018:vi23.
Komotar 2010 {published data only}
-
- Komotar RJ, Starke RM, Connolly ES, Sisti MB. Evaluating the benefit of repeat surgery for recurrent glioblastoma multiforme. Neurosurgery 2010;67(6):N16-7. - PubMed
Konkel 2018 {published data only}
-
- Konkel B, Caflisch L, Duque AED, Brenner AJ. Updated results from a prospective, randomized phase 2 study in patients with first relapse of high-grade astrocytoma using TVB-2640 in combination with avastin versus avastin alone. Neuro-oncology. Conference abstract of the Society for Neuro-Oncology 2018;20:vi16.
-
- Konkel B, Caflisch LD, Duque AED, Michalek J, Liu Q, Brenner AJ. Prospective phase II trial in patients with first relapse of high-grade astrocytoma using TVB-2640 in combination with bevacizumab versus bevacizumab alone. Journal of Clinical Oncology 2019;37(15 suppl 1):2064.
-
- NCT03032484. TVB- 2640 in combination with bevacizumab in patients with first relapse of high grade astrocytoma. ClinicalTrials.gov 2017.
Lang 2018 {published data only}
-
- Lang FF, Tran ND, Puduvalli VK, Elder JB, Fink KL, Conrad CA, et al. Phase 1b open-label randomized study of the oncolytic adenovirus DNX-2401 administered with or without interferon gamma for recurrent glioblastoma. Journal of Clinical Oncology 2017;35(15 suppl):2002.
-
- Tufaro F, Peterkin J, Gammon K, Salvosa M, Mitchell E, Ewald B, et al. Phase 1B open-label randomized study of the oncolytic virus DNX-2401 administered with or without interferon gamma for recurrent glioblastoma. In: Neuro-oncology. Conference abstract. Vol. 18. 2016:vi24.
Levin 2017 {published data only}
-
- Levin VA, Cruickshank S. STELLAR: a phase 3, randomized, open-label study of eflornithine with lomustine vs lomustine for patients with first recurrence of anaplastic astrocytoma after RT and adjuvant temozolomide. Journal of Clinical Oncology. ASCO conference abstract. 2017;35(15 suppl):10.1200/JCO.2017.35.15_suppl.TPS2081.
-
- NCT02796261. Study to evaluate eflornithine and lomustine vs lomustine in recurrent anaplastic astrocytoma (AA) patients. ClinicalTrials.gov 2016.
Mau‐Sorensen 2016 {published data only}
-
- Karyopharm Therapeutics Inc. A phase 2 study evaluating the efficacy and safety of selinexor (KPT-330) in patients with recurrent gliomas. ClinicalTrials.gov/ct2/show/NCT01986348 2013.
-
- Lassen UN, Mau-Soerensen M, Kung AL, Wen PY, Lee EQ, Plotkin SR, et al. A phase 2 study on efficacy, safety and intratumoral pharmacokinetics of oral selinexor (KPT-330) in patients with recurrent glioblastoma (GBM). Journal of Clinical Oncology 2015;33(15):SUPPL. 1.
-
- Mau-Sorensen M, Plotkin SR, Wen PY, Kung AL, Lassen UN, Saint-Martin JR, et al. A phase 2 study on efficacy, safety and intratumoral pharmacokinetics of oral selinexor (KPT-330) in patients with recurrent glioblastoma (GBM). In: Journal of Clinical Oncology. Conference abstract. Vol. 34. 2016:2077.
Minniti 2015 {published data only}
-
- Minniti G, Agolli L, Falco T, Scaringi C, Lanzetta G, Caporello P, et al. Hypofractionated stereotactic radiotherapy in combination with bevacizumab or fotemustine for patients with progressive malignant gliomas. Journal of Neuro-oncology 2015;122(3):559-66. - PubMed
Muhic 2013 {published data only}
-
- Muhic A, Poulsen HS, Sorensen M, Grunnet K, Lassen U. Phase II open-label study of nintedanib in patients with recurrent glioblastoma multiforme. Journal of Neuro-oncology 2013;111(2):205-12. - PubMed
NCT02529072 2015 {published data only}
-
- NCT02529072. Nivolumab with DC vaccines for recurrent brain tumors. ClinicalTrials.gov 2015.
NCT02852655 2016 {published data only}
-
- NCT02852655. A pilot surgical trial to evaluate early immunologic pharmacodynamic parameters for the PD-1 checkpoint inhibitor, pembrolizumab (MK-3475), in patients with surgically accessible recurrent/progressive glioblastoma. ClinicalTrials.gov 2016.
-
- Prins R, Mochizuki A, Orpilla J, Lee A, Davidson T, Gaffey S, et al. Neoadjuvant anti-PD-1 immunotherapy promotes intratumoral and systemic immune responses in recurrent glioblastoma: an ivy consortium trial. In: Neuro-oncology. Vol. Conference: 23rd Annual Scientific Meeting and Education Day of the Society for Neuro-oncology. United States. 20. 2018:vi3.
NCT02866747 2016 {published data only}
-
- NCT02866747. A study evaluating the association of hypofractionated stereotactic radiation therapy and durvalumab for patients with recurrent glioblastoma. ClinicalTrials.gov 2016.
NCT03014804 2016 {published data only}
-
- NCT03014804. Autologous dendritic cells pulsed with tumor lysate antigen vaccine and nivolumab in treating patients with recurrent glioblastoma. ClinicalTrials.gov 2016.
NCT03149575 2017 {published data only}
-
- NCT03149575. VAL-083 Phase 3 Study in Temozolomide-Avastin (Bevacizumab) Recurrent GBM. ClinicalTrials.gov 2017.
Penas‐Prado 2015 {published data only}
-
- Penas-Prado M, Hess KR, Levin VA, De Groot JF, Colman H, Groves MD, et al. Phase I study of vorinostat combined with isotretinoin and temozolomide in adults with recurrent malignant gliomas. Journal of Clinical Oncology 2015;33(15):SUPPL. 1.
Rahman 2014 {published data only}
Reardon 2008 {published data only}
-
- Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O'Neill A, Plotkin S, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. Journal of Clinical Oncology 2008;26(34):5610-7. - PubMed
Ruiz‐Sanchez 2012 {published data only}
-
- Ruiz-Sanchez D, Calero MA, Sastre-Heres AJ, Garcia MTI, Hernandez MAC, Martinez FM, et al. Effectiveness of the bevacizumab-irinotecan regimen in the treatment of recurrent glioblastoma multiforme: comparison with other second-line treatments without this regimen. Oncology Letters 2012;4(5):1114-8. - PMC - PubMed
Schmidt 2006 {published data only}
-
- Schmidt F, Fischer J, Herrlinger U, Dietz K, Dichgans J, Weller M PCV chemotherapy for recurrent glioblastoma. PCV chemotherapy for recurrent glioblastoma. Neuro-oncology 2006;66(4):587-9. - PubMed
Seystahl 2013 {published data only}
-
- Seystahl K, Wiestler B, Hundsberger T, Happold C, Wick W, Weller M, et al. Bevacizumab alone or in combination with irinotecan in recurrent WHO Grade II and Grade III gliomas. European Neurology 2013;69(2):95-101. - PubMed
Short 2017 {published data only}
-
- Short SC, Little C. A 2-part safety and exploratory efficacy randomised double-blind, placebo-controlled study of a 1:1 ratio of cannabidiol and delta-9-tetrahydrocannabinol (CBD: tHC) plus dose intense temozolomide in patients with recurrent glioblastoma multiforme (GBM). Neuro-oncology 2017;Conference: 22nd Annual Scientific Meeting and Education Day of the Society for Neuro-oncology. United States. 19:vi13.
Socha 2016 {published data only}
-
- Socha J, Kepka L, Ghosh S, Roa W, Kumar N, Sinaika V, et al. Outcome of treatment of recurrent glioblastoma multiforme in elderly and/or frail patients. Journal of Neuro-oncology 2016;126(3):493-8. - PubMed
Sun 2013 {published data only}
-
- Sun J, Yang XJ, Yang SY. Multicenter randomized controlled study of temozolomide versus semustine in the treatment of recurrent malignant glioma]. [Chinese CNO - CN-00908562. Zhonghua yi xue za zhi 2013;93(3):165-8. - PubMed
Taylor 2018 {published data only}
Van den Bent 2009 {published data only}
-
- den Bent MJ, Brandes AA, Rampling R, Kouwenhoven MC, Kros JM, Carpentier AF, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. Journal of Clinical Oncology 2013;27 XST - M XAOC - TRT(8):1268-74. - PMC - PubMed
van den Bent 2016 {published data only}
-
- NCT03149003. A study of DSP-7888 dosing emulsion in combination with bevacizumab in patients with recurrent or progressive glioblastoma following initial therapy. ClinicalTrials.gov 2017.
-
- den Bent M, Clement P, Vos F, Platten M, Mulholland P, Taphoorn M, et al. Clinical results of the eortc randomized phase ii tavarec trial on temozolomide with or without bevacizumab in 1st recurrence of grade ii or III glioma without 1P/19Q co-deletion. Neuro-oncology 2016;Conference: 21st Annual Scientific Meeting and Education Day of the Society for Neuro-oncology. United States 18:vi3.
Vauleon 2012 {published data only}
-
- Vauleon E, Mesbah H, Gedouin D, Lecouillard I, Louvel G, Hamlat A, et al. Retrospective analysis of 24 recurrent glioblastoma after chemoradiation and treated with nitrosoureas or irinotecan and bevacizumab. Bulletin du Cancer 2012;99(2):121-6. - PubMed
Weller 2015 {published data only}
-
- Weller M, Tabatabai G, Kastner B, Felsberg J, Steinbach JP, Wick A, et al. MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR Trial. Clinical Cancer Research 2015;21(9):2057-64. - PubMed
Yasuda 2018 {published data only}
-
- Yasuda T, Muragaki Y, Nitta M, Miyamoto K, Oura Y, Henmi T, et al. Effectiveness of stereotactic radiotherapy and bevacizumab for recurrent high-grade gliomas: A potential therapy for isocitrate dehydrogenase wild-type recurrent high-grade gliomas. World Neurosurgery 2018;epub. doi 10.1016/j.wneu.2018.03.161:e1138. - PubMed
Yung 2000 {published data only}
Zadeh 2018 {published data only}
-
- NCT02414165. The toca 5 trial: toca 511 & toca FC versus standard of care in patients with recurrent high grade glioma. NCT02414165 2015.
-
- Zadeh G, Walbert T, Perry J, Zhu J, Salacz M, Bota D, et al. Toca 5: toca 511 combined with Toca FC versus standard of care in patients undergoing planned resection for recurrent glioblastoma or anaplastic astrocytoma. In: Canadian Journal of Neurosciences. Vol. 45. 2018:S2.
Zakharia 2017 {published data only}
-
- Zakharia Y, Munn D, Link C, Vahanian N, Kennedy E. Interim analysis of phase 1B/2 combination study of the IDO pathway inhibitor indoximod with temozolomide for adult patients with temozolomide-refractory primary malignant brain tumors. Neuro-oncology 2017;18:vi13-4.
References to ongoing studies
ACTRN12617000534381 2017 {published data only}
ChiCTR1900020646 2019 {published data only}
-
- ChiCTR1900020646. A multicenter open-label randomized controlled trial for apatinib combined with temozolomide in adult primary glioblastoma at first recurrence. ChiCTR1900020646 2019.
Combs 2010 {published data only}
-
- Combs SE, Burkholder I, Edler L, Rieken S, Habermehl D, Jakel O, et al. Randomised phase I/II study to evaluate carbon ion radiotherapy versus fractionated stereotactic radiotherapy in patients with recurrent or progressive gliomas: the CINDERELLA trial. BMC Cancer 2010;10:533. [https://doi.org/10.1186/1471-2407-10-533] - PMC - PubMed
JCOG1308C 2019 {published data only}
-
- jRCTs031180083. JCOG1308C: a multicenter randomized phase III study for recurrent glioblastoma. jRCTs031180083 2019.
KCT0002632 2018 {published data only}
-
- KCT0002632. Phase 2 clinical trial for the efficacy and safety of low dose Temozolomide plus metformin as combination chemotherapy compared with low dose Temozolomide plus placebo in patient with recurrent or refractory Glioblastoma. CRIS_Clinical Search Information Service 2018.
NCT01252459 2016 {published data only}
-
- Oehlke O, Mix M, Graf E, Schimek-Jasch T, Nestle U, Gotz I, et al. Amino-acid PET versus MRI guided re-irradiation in patients with recurrent glioblastoma multiforme (GLIAA) - protocol of a randomized phase II trial (NOA 10/ARO 2013-1). ClinicalTrials.gov 2016;16(1):769. - PMC - PubMed
NCT01903330 2018 {published data only}
-
- Bota D, Carrillo J, Kong XT, Fu D, Chung J, Pretto C, et al. A randomized, double-blinded, placebocontrolled phase 2 study of the ERC-1671 (Gliovac) vaccine in combination with bevacizumab (BEV) in recurrent GBM patients: safety lead-in analysis. Neuro-oncology 2016;Conference: 21st Annual Scientific Meeting and Education Day of the Society for Neuro-Oncology. United States.18:vi22.
-
- Bota D, Chung J, Carrillo J, Kong XT, Fu B, Pretto C, et al. A preliminary data report on a phase 2 study of ERC1671 in recurrent glioblastoma. Neuro-oncology. Conference abstract of Society for Neuro-oncology 2017;19(Suppl 6):vi109. [DOI: 10.1093/neuonc/nox168.448] - DOI
-
- Bota DA, Chung J, Dandekar M, Carrillo JA, Kong XT, Fu BD, et al. Phase II study of ERC1671 plus bevacizumab versus bevacizumab plus placebo in recurrent glioblastoma: interim results and correlations with CD4+ T-lymphocyte counts. CNS Oncology 2018;7(3):CNS22. [DOI: 10.2217/cns-2018-0009] - DOI - PMC - PubMed
-
- Bota DA, Chung J, Dandekar M, Carrillo JA, Kong XT, Fu DB, et al. Phase 2 study of erc1671 plus bevacizumab vs bevacizumab plus placebo in recurrent GBM interim results and correlations with CD4+ T lymphocyte counts. Neuro-oncology. Conference abstract of Society for Neuro-oncology 2018;20:vi7. - PMC - PubMed
NCT02394626 2015 {published data only}
-
- NCT02394626. Surgery for Recurrent Glioblastoma. ClinicalTrials.gov 2015.
NCT02678975 2016 {published data only}
-
- NCT02678975. Disulfiram in Recurrent Glioblastoma. ClinicalTrials.gov 2016.
NCT02715297 2018 {published data only}
-
- NCT02715297. Adjuvant stereotactic fractionated radiotherapy to the resection cavity in recurrent glioblastoma. ClinicalTrials.gov 2016.
NCT02761070 2016 {published data only}
-
- NCT02761070. Bevacizumab alone versus dose-dense temozolomide followed by bevacizumab for recurrent glioblastoma, phase III. ClinicalTrials.gov 2016.
NCT02794883 2016 {published data only}
-
- NCT02794883. Tremelimumab and durvalumab in combination or alone in treating patients with recurrent malignant glioma. ClinicalTrials.gov 2016.
NCT02942264 2016 {published data only}
-
- NCT02942264. TG02 plus dose-dense or metronomic temozolomide followed by randomized phase II trial of TG02 plus temozolomide versus temozolomide alone in adults with recurrent anaplastic astrocytoma and glioblastoma. ClinicalTrials.gov 2016.
NCT02974621 2016 {published data only}
-
- NCT02974621. Cediranib maleate and olaparib compared to bevacizumab in treating patients with recurrent glioblastoma. ClinicalTrials.gov 2016.
NCT03025893 2019 {published data only}
-
- Brahm CG, Van Linde ME, Labots M, Kouwenhoven MC, Aliaga ES, Enting RH, et al. A phase II/III trial of high-dose, intermittent sunitinib in patients with recurrent glioblastoma: the STELLAR study. In: Cancer Research. Vol. 79. 2019.
-
- EUCTR2016-001797-15-NL. A trial testing an alternative high-dose, intermittend scheduling for sunitinib in patients with recurrent brain cancer. European Clinical Trials Register 2016.
-
- NCT03025893. A phase II/III study of high-dose, intermittent sunitinib in patients with recurrent glioblastoma multiforme. ClinicalTrials.gov 2017.
-
- NCTR6308. A phase II/III study of high-dose, intermittent sunitinib in patients with recurrent glioblastoma multiforme: the STELLAR study. ClinicalTrials.gov 2016.
NCT03149003 2018 {published data only}
-
- De Groot JF, Cloughesy TF, Pitz MW, Narita Y, Nonomura T. A randomized, multicenter phase 2 study of DSP-7888 dosing emulsion in combination with bevacizumab (Bev) versus Bev alone in patients with recurrent or progressive glioblastoma. ClinicalTrials.gov 2018;36(15).
NCT03632135 2018 {published data only}
-
- NCT03632135. Standard chemotherapy vs. chemotherapy guided by cancer stem cell test in recurrent glioblastoma. ClinicalTrials.gov 2018.
NCT03746288 2018 {published data only}
-
- NCT03746288. To evaluate the efficacy and safety of CAN008 combined with re-irradiation (rRT) for treating patients with recurrent glioblastoma (GBM). ClinicalTrials.gov 2018.
NCT03970447 2019b {published data only}
-
- NCT03970447. A trial to evaluate multiple regimens in newly diagnosed and recurrent glioblastoma. ClinicalTrials.gov 2019.
NCT04003649 2019 {published data only}
-
- NCT04003649. IL13Ralpha2-targeted chimeric antigen receptor (CAR) T cells with or without nivolumab and ipilimumab in treating patients with recurrent or refractory glioblastoma. ClinicalTrials.gov 2019.
Additional references
Ameratunga 2018
Barrios 2012
-
- Barrios JMR, Alcántara FP, Palomo CC, García PG, De Las Heras AE, Riestra BM. The use of cost per life year gained as a measurement of cost-effectiveness in Spain: a systematic review of recent publications. European Journal of Health Economics 2012;13(6):723-40. - PubMed
Batchelor 2006
-
- Batchelor TT, Byrne TN. Supportive care of brain tumor patients. Hematology/oncology Clinics of North America 2006;20(6):1337-61. - PubMed
Brown 2018
Chaimani 2015
-
- Chaimani A, Salanti G. Visualizing assumptions and results in network meta‐analysis: the network graphs package. Stata Journal 2015;15(4):905-50.
Chapman 2019
Conen 2017
-
- Conen KL, Matter-Walstra K, Schadelin S, Mariani L, Hess V. Benefits and costs of bevacizumab in recurrent glioblastoma: a quality adjusted survival and cost analysis (EVALUATE). In: Journal of Clinical Oncology, Conference: 2017 Annual Meeting of the American Society of Clinical Oncology, ASCO. United States. 35. Vol. 35. 2017.
CRUK 2020
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Chapter 15: Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition. London: BMJ Publication Group, 2001.
Drugs.com
-
- Drugscom. Alkylating agents. www.drugs.com/drug-class/alkylating-agents.html (accessed 9 December 2019).
Dumitru 2018
EANO 2017
-
- Pace A, Dirven L, Koekkoek JAF, Golla H, Fleming J, Ruda R. European association for Neuro-oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncology 2017;18(6):e330-40. - PubMed
Easaw 2011
Efthimiou 2016
-
- Efthimiou O, Debray TP, Valkenhoef G, Trelle S, Panayidou K, Moons KG, et al. GetReal in network meta-analysis: a review of the methodology. Research Synthesis Methods 2016;7(3):236-63. - PubMed
EPOC 2015
-
- Cochrane Effective Practice and Organisation of Care (EPOC). EPOC resources for review authors. 2015. epoc.cochrane.org/epoc-specific-resources-review-authors (accessed 6 July 2016).
Higgins 2003
Higgins 2019
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 [updated July 2019]. Cochrane, 2019. Available from www.training.cochrane.org/handbook.
JLA 2015
-
- MacDonald L, on behalf of the Neuro-oncology Group. Top 10 priorities for clinical research in primary brain and spinal cord tumours. www.jla.nihr.ac.uk/priority-setting-partnerships/neuro-oncology/download... (accessed 1 March 2018).
Kazmi 2019
-
- Kazmi F, Soon YY, Leong YH, Koh WY, Vellayappan B. Re-irradiation for recurrent glioblastoma (GBM): a systematic review and meta-analysis. Journal of Neuro-oncology 2019;142:79-90. - PubMed
Kim 2019
Lombardi 2017
-
- Lombardi G, Pambuku A, Bellu L, Farina M, Della Puppa A, Denaro L, et al. Effectiveness of antiangiogenic drugs in glioblastoma patients: A systematic review and meta-analysis of randomised clinical trials. Critical Reviews in Oncology/hematology 2017;111:94-102. - PubMed
Malmstrom 2012
-
- Malmstrom A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncology 2012;13(9):916-26. - PubMed
Mandl 2008
-
- Mandl ES, Dirven CM, Buis DR, Postma TJ, Vandertop WP. Repeated surgery for glioblastoma multiforme: only in combination with other salvage therapy. Surgical Neurology 2008;69(5):506-9. - PubMed
Martin‐McGill 2018
MASCC 2019
-
- Multinational Association of Supportive Care in Cancer. What is supportive care. www.mascc.org/about-mascc (accessed 8 October 2019).
Messali 2014
-
- Messali A, Villacorta R, Hay JW. A review of the economic burden of glioblastoma and the cost effectiveness of pharmacologic treatments. PharmacoEconomics 2014;32:1201-12. - PubMed
NCCN 2018
-
- National Comprehensive Cancer Network. NCCN Guidelines version 1. 2018. Central Nervous System Cancers. www.optune.com/Content/pdfs/CNS_FlashCard_4Page.pdf (accessed 5 April 2020).
NICE 2018
-
- National Institute for Health and Care Excellence. Brain tumours (primary) and brain metastases in adults. Available at www.nice.org.uk/guidance/ng99. - PubMed
Niyazi 2011
-
- Niyazi M, Siefert A, Schwarz SB, Ganswindt U, Kreth FW, Tonn JC, et al. Therapeutic options for recurrent malignant glioma. Radiotherapy and Oncology 2011;98(1):1-14. - PubMed
Parasramka 2017
Perry 2017
-
- Perry JR, Laperriere N, O'Callaghan CJ, Brandes AA, Menten J, Phillips C, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma CNO - CN-01366980. New England Journal of Medicine 2017;376(11):1027-37. - PubMed
Review Manager 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roussakow 2017
-
- Roussakow SV. Clinical and economic evaluation of modulated electrohyperthermia concurrent to dose-dense temozolomide 21/28 days regimen in the treatment of recurrent glioblastoma: a retrospective analysis of a two-centre German cohort trial with systematic comparison and effect-to-treatment analysis. BMJ Open 2017;7:e017387. - PMC - PubMed
Ruiz‐Sanchez 2016
Schünemann 2019
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors), editors(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Wiley, 2019:Available from training.cochrane.org/handbook/current/chapter-15 (Accessed 5 April 2020).
Shemilt 2019
-
- Shemilt I, Aluko P, Graybill E, Craig D, Henderson C, Drummond M, et al, on behalf of the Campbell and Cochrane Economics Methods Group. Chapter 20: Economic evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Sterne 2016
Stupp 2005
-
- Stupp R, Mason WP, den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine 2005;352(10):987-96. - PubMed
Thompson 2019
Thon 2013
Voigt 2016
White 2015
-
- White IR. Network meta‐analysis. Stata Journal 2015;15(4):951-85.
Wick 2012
-
- Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncology 2012;13(7):707-15. - PubMed
Wong 1999
-
- Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. Journal of Clinical Oncology 1999;17(8):2572-8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

