Paracetamol/acetaminophen (single administration) for perineal pain in the early postpartum period
- PMID: 34559424
- PMCID: PMC8094229
- DOI: 10.1002/14651858.CD008407.pub3
Paracetamol/acetaminophen (single administration) for perineal pain in the early postpartum period
Abstract
Background: Perineal pain is a common but poorly studied adverse outcome following childbirth. Pain may result from perineal trauma due to bruising, spontaneous tears, surgical incisions (episiotomies), or in association with operative vaginal births (ventouse or forceps-assisted births). This is an update of a review last published in 2013.
Objectives: To determine the efficacy of a single administration of paracetamol (acetaminophen) used in the relief of acute postpartum perineal pain.
Search methods: For this update, we searched the Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (9 December 2019), and reference lists of retrieved studies.
Selection criteria: Randomised controlled trials (RCTs), including cluster-RCTs, comparing paracetamol to placebo. We excluded quasi-RCTs and cross-over trials. Data from abstracts would be included only if authors had confirmed in writing that the data to be included in the review had come from the final analysis and would not change.
Data collection and analysis: Two review authors assessed each study for inclusion and extracted data. One review author reviewed the decisions and confirmed calculations for pain relief scores. We assessed the certainty of the evidence using the GRADE approach.
Main results: This update identified no new trials so the results remain unchanged. However, by applying the GRADE assessment of the evidence, the interpretation of main results differed from previous version of this review. We identified 10 studies involving 2044 women, but all these studies involved either three or four groups, looking at differing drugs or doses. We have only included the 1301 women who were in the paracetamol versus placebo arms of the studies. Of these, five studies (482 women) assessed 500 mg to 650 mg and six studies (797 women) assessed 1000 mg of paracetamol. One study assessed 650 mg and 1000 mg compared with placebo and contributed to both comparisons. We used a random-effects meta-analysis because of the clinical variability among studies. Studies were from the 1970s to the early 1990s, and there was insufficient information to assess the risk of bias adequately, hence the findings need to be interpreted within this context. The certainty of the evidence for the two primary outcomes on which data were available was assessed as low, downgraded for overall unclear risk of bias and for heterogeneity (I² statistic 60% or greater). More women may experience pain relief with paracetamol compared with placebo (average risk ratio (RR) 2.14, 95% confidence interval (CI) 1.59 to 2.89; 10 trials, 1279 women), and fewer women may need additional pain relief with paracetamol compared with placebo (average RR 0.34, 95% CI 0.21 to 0.55; 8 trials, 1132 women). However, the certainty of the evidence was low, downgraded for unclear overall risk of bias and substantial heterogeneity. One study used the higher dose of paracetamol (1000 mg) and reported maternal drug adverse effects. There may be little or no difference in the incidence of nausea (average RR 0.18, 95% CI 0.01 to 3.66; 1 trial, 232 women; low-certainty evidence), or sleepiness (average RR 0.89, 95% CI 0.18 to 4.30; 1 trial, 232 women; low-certainty evidence). No other maternal adverse events were reported. None of the studies assessed neonatal drug adverse effects.
Authors' conclusions: A single dose of paracetamol may improve perineal pain relief following vaginal birth, and may reduce the need for additional pain relief. Potential adverse effects for both women and neonates were not appropriately assessed. Any further trials should also address the gaps in evidence concerning maternal outcomes such as satisfaction with postnatal care, maternal functioning/well-being (emotional attachment, self-efficacy, competence, autonomy, confidence, self-care, coping skills) and neonatal drug adverse effects.
บทนำ: อาการปวดฝีเย็บเป็นผลข้างเคียงที่พบบ่อยหลังการคลอดบุตร แต่มีการศึกษาไม่ดี ความเจ็บปวดอาจเป็นผลมาจากการบาดเจ็บของฝีเย็บอันเนื่องมาจากรอยฟกช้ำ การฉีกขาดที่เกิดขึ้นเอง การตัด (ตัดฝีเย็บ) หรือเกี่ยวกับการคลอดทางช่องคลอด (การคลอดด้วยเครื่องดูดหรือด้วยคีม) นี่คือการอัปเดตของการทบทวนวรรณกรรมที่เผยแพร่ล่าสุดในปี 2013 วัตถุประสงค์: เพื่อประเมินประสิทธิภาพของการใช้ยาพาราเซตามอล (acetaminophen) เพียงครั้งเดียวที่ใช้ในการบรรเทาอาการปวดฝีเย็บเฉียบพลันหลังคลอด วิธีการสืบค้น: สำหรับการอัปเดตนี้เราได้ค้นหา Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (9 ธันวาคม 2019) และรายการอ้างอิงของการศึกษาที่พบ เกณฑ์การคัดเลือก: Randomized controlled trials (RCTs) รวมทั้ง cluster‐RCTs เปรียบเทียบพาราเซตามอลกับยาหลอก การวิจัยแบบ Quasi‐RCTs และ cross‐over trials ไม่ได้รวมเข้ามาในการทบทวนวรรรกรรมครั้งนี้ ข้อมูลจากบทคัดย่อจะรวมเฉพาะในกรณีที่ผู้เขียนยืนยันเป็นลายลักษณ์อักษรว่าข้อมูลที่จะรวมในการทบทวนวรรณกรรมนั้นมาจากการวิเคราะห์ขั้นสุดท้ายและจะไม่เปลี่ยนแปลง การรวบรวมและวิเคราะห์ข้อมูล: ผู้วิจัย 2 คนได้ทำการประเมินงานวิจัยเพื่อการคัดเข้า และคัดลอกข้อมูลอย่างเป็นอิสระต่อกัน ผู้วิจัย 1 คน ได้ตรวจสอบการตัดสินใจ และยืนยันการคำนวณคะแนนการบรรเทาอาการปวด และประเมินความเชื่อมั่นของหลักฐานโดยวิธีแนวทางของ GRADE ผลการวิจัย: การอัปเดตนี้ระบุว่าไม่มีการทดลองใหม่ดังนั้นผลลัพธ์จึงไม่เปลี่ยนแปลง อย่างไรก็ตามด้วยการใช้การประเมิน GRADE ของหลักฐาน การตีความผลลัพธ์หลักแตกต่างจากการทบทวนวรรณกรรมรุ่นก่อนหน้านี้ เราพบการศึกษา 10 รายการที่เกี่ยวข้องกับสตรี 2044 คน แต่การศึกษาทั้งหมดนี้เกี่ยวข้องกับ 3 หรือ 4 กลุ่มโดยพิจารณายาหรือขนาดที่ต่างกัน เราได้รวมเฉพาะสตรี 1301 คนที่อยู่ในกลุ่มยาพาราเซตามอลเทียบกับยาหลอกในการศึกษาเหล่านี้ ในจำนวนนี้ มีการศึกษา 5 รายการ (สตรี 482 คน) ประเมิน 500 มก. ถึง 650 มก. และการศึกษา 6 รายการ (สตรี 797 คน) ประเมินพาราเซตามอล 1,000 มก. การศึกษาหนึ่งรายการประเมิน 650 มก. และ 1,000 มก. เมื่อเทียบกับยาหลอก และมีส่วนในการเปรียบเทียบทั้งสอง เราใช้ random‐effects meta‐analysis เนื่องจากความแปรปรวนทางคลินิกระหว่างการศึกษา การศึกษาตั้งแต่ทศวรรษ 1970 ถึงต้นทศวรรษ 1990 และมีข้อมูลไม่เพียงพอที่จะประเมินความเสี่ยงของอคติได้อย่างเพียงพอ ดังนั้นจึงจำเป็นต้องตีความข้อค้นพบภายในบริบทนี้ ความเชื่อมั่นของหลักฐานสำหรับผลลัพธ์หลัก 2 อย่าง ที่มีข้อมูลอยู่นั้นได้รับการประเมินว่าต่ำ โดยลดระดับลงสำหรับความเสี่ยงที่ไม่ชัดเจนโดยรวมของอคติและความแตกต่างกัน ( สถิติ I² 60% ขึ้นไป) สตรีจำนวนมากกว่า อาจได้รับการบรรเทาอาการปวดด้วยพาราเซตามอลเมื่อเทียบกับยาหลอก (อัตราส่วนความเสี่ยงโดยเฉลี่ย (RR) 2.14, ช่วงความเชื่อมั่น 95% (CI) 1.59 ถึง 2.89; การทดลอง 10 รายการ, สตรี 1279 คน) และสตรีจำนวนน้อยกว่าอาจต้องการยาบรรเทาอาการปวดเพิ่มเติม ในกลุ่มพาราเซตามอลเมื่อเทียบกับยาหลอก (เฉลี่ย RR 0.34, 95% CI 0.21 ถึง 0.55; การทดลอง 8 รายการ, สตรี 1132 คน) อย่างไรก็ตาม ความเชื่อมั่นของหลักฐานอยู่ในระดับต่ำ โดยลดระดับลงเนื่องจากความเสี่ยงโดยรวมของอคติที่ไม่ชัดเจน และความแตกต่างอย่างมีนัยสำคัญ การศึกษาหนึ่งรายการ ใช้พาราเซตามอลในปริมาณที่สูงขึ้น (1,000 มก.) และรายงานผลข้างเคียงของยาในมารดา อุบัติการณ์ของอาการคลื่นไส้อาจมีความแตกต่างกันเพียงเล็กน้อยหรือไม่แตกต่าง (ค่าเฉลี่ย RR 0.18, 95% CI 0.01 ถึง 3.66; การทดลอง 1 รายการ, สตรี 232 คน; หลักฐานความเชื่อมั่นต่ำ) หรือง่วงนอน (RR เฉลี่ย 0.89, 95% CI 0.18 ถึง 4.30; การทดลอง 1 รายการ, สตรี 232 คน; หลักฐานความเชื่อมั่นต่ำ) ไม่มีรายงานเหตุการณ์ไม่พึงประสงค์อื่น ๆ ของมารดา ไม่มีการศึกษาใดที่ประเมินผลข้างเคียงของยาในทารกแรกเกิด ข้อสรุปของผู้วิจัย: ยาพาราเซตามอลเพียงครั้งเดียวอาจช่วยบรรเทาอาการปวดฝีเย็บหลังคลอดทางช่องคลอดและอาจลดความจำเป็นในการบรรเทาอาการปวดเพิ่มเติม ผลข้างเคียงที่อาจเกิดขึ้นต่อทั้งสตรีและทารกแรกเกิดไม่ได้รับการประเมินอย่างเหมาะสม การทดลองเพิ่มเติมใด ๆ ควรจัดการช่องว่างของหลักฐานเกี่ยวกับผลลัพธ์ของมารดา เช่นความพึงพอใจต่อการดูแลหลังคลอดการทำงานของมารดา / ความเป็นอยู่ที่ดี (ความผูกพันทางอารมณ์ ความสามารถในตนเอง ความสามารถ ความเป็นอิสระความมั่นใจการดูแลตนเอง ทักษะการเผชิญปัญหา) และผลกระทบของยาต่อทารกแรกเกิด.
پیشینه: درد پرینه (perineal) شایع است اما پیامد جانبی آن پس از زایمان بهطور ضعیفی مورد مطالعه قرار گرفته است. درد ممکن است ناشی از ترومای پرینه به علت کبودی، پارگیهای خودبهخودی، برشهای جراحی (اپیزیوتومیها) یا ناشی از زایمان واژینال اوپراتیو (زایمانهایی با کمک ونتوس یا فورسپس) باشد. این یک بهروزرسانی از مروری است که آخرین بار در سال 2013 منتشر شد. اهداف: تعیین اثربخشی تجویز تک‐دوز از پاراستامول (استامینوفن) برای تسکین درد حاد پرینه پس از زایمان. روشهای جستوجو: برای این بهروزرسانی، پایگاه ثبت کارآزماییهای گروه بارداری و زایمان در کاکرین، ClinicalTrials.gov؛ پلتفرم بینالمللی پایگاه ثبت کارآزماییهای بالینی سازمان جهانی بهداشت (9 دسامبر 2019)، و فهرست منابع مطالعات بازیابی شده را جستوجو کردیم. معیارهای انتخاب: کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs)، شامل RCTهای خوشهای، که به مقایسه پاراستامول با دارونما (placebo) پرداختند. شبه‐RCTها و کارآزماییهای متقاطع (cross‐over) را خارج کردیم. دادههای بهدست آمده از چکیده مقالات فقط در صورتی وارد شدند که نویسندگان بهصورت کتبی تائید کرده باشند که دادههایی که باید در این مرور وارد شوند از تجزیهوتحلیل نهایی بهدست آمده و تغییر نمیکنند. گردآوری و تجزیهوتحلیل دادهها: دو نویسنده مرور هر مطالعه را برای ورود ارزیابی و دادهها را استخراج کردند. یک نویسنده مرور، تصمیمات را بررسی و محاسبات انجام شده را برای نمرات تسکین درد تائید کرد. قطعیت شواهد را با استفاده از رویکرد درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) ارزیابی کردیم. نتایج اصلی: این بهروزرسانی هیچ کارآزمایی جدیدی را شناسایی نکرد بنابراین نتایج بدون تغییر باقی میمانند. با این حال، با استفاده از ارزیابی GRADE از شواهد، تفسیر نتایج اصلی متفاوت از نسخه قبلی این مرور بود. ما 10 مطالعه را شامل 2044 زن شناسایی کردیم، اما همه این مطالعات شامل سه یا چهار گروه بودند، که داروها یا دوزهای مختلف را بررسی میکردند. ما فقط 1301 زن را وارد کردیم که در این مطالعات در بازوی پاراستامول در برابر بازوی دارونما قرار داشتند. از این تعداد، پنج مطالعه (482 زن) دوزهای 500 میلیگرم تا 650 میلیگرمی و شش مطالعه (797 زن) دوز 1000 میلیگرمی را از پاراستامول ارزیابی کردند. یک مطالعه دوز 650 میلیگرم و 1000 میلیگرم را در مقایسه با دارونما ارزیابی کرده و در هر دو مقایسه مشارکت داشت. به دلیل تنوع بالینی میان مطالعات، از متاآنالیز اثرات تصادفی استفاده کردیم. مطالعات مربوط به دهه 1970 تا اوایل دهه 1990 بودند و اطلاعات کافی را برای ارزیابی کافی خطر سوگیری (bias) ارائه ندادند، از این رو یافتهها باید در این زمینه تفسیر شوند. قطعیت شواهد برای دو پیامد اولیه که دادههای آنها در دسترس بودند در سطح پائین ارزیابی شد، که دلیل آن، خطر کلی سوگیری نامشخص و ناهمگونی سطح شواهد بود (آماره I2 معادل 60% یا بیشتر). زنان بیشتری ممکن است به تسکین درد با پاراستامول در مقایسه با دارونما دست یابند (میانگین خطر نسبی (RR): 2.14؛ 95% فاصله اطمینان (CI): 1.59 تا 2.89؛ 10 کارآزمایی، 1279 زن)، و احتمالا زنان کمتری با پاراستامول در مقایسه با دارونما نیاز به تجویز ضد‐درد بیشتر خواهند داشت (میانگین RR؛ 0.34؛ 95% CI؛ 0.21 تا 0.55؛ 8 کارآزمایی، 1132 زن). با این حال، به دلیل خطر کلی نامشخص سوگیری و ناهمگونی قابل توجه، سطح قطعیت شواهد پائین بود. در یک مطالعه از دوز بالاتر پاراستامول (1000 میلیگرم) استفاده شده و عوارض جانبی دارو در مادر گزارش شد. ممکن است تفاوتی اندک یا عدم تفاوت در بروز تهوع (میانگین RR؛ 0.18؛ 95% CI؛ 0.01 تا 3.66؛ 1 کارآزمایی، 232 زن؛ شواهد با قطعیت پائین)، یا خوابآلودگی (میانگین RR؛ 0.89؛ 95% CI؛ 0.18 تا 4.30؛ 1 کارآزمایی، 232 زن؛ شواهد با قطعیت پائین) وجود داشته باشد. هیچ موردی از حوادث جانبی در مادر گزارش نشد. در هیچ یک از مطالعات عوارض جانبی دارو در نوزادان ارزیابی نشد. نتیجهگیریهای نویسندگان: یک تک‐دوز از پاراستامول ممکن است باعث تسکین درد پرینه پس از زایمان واژینال شود، و میتواند نیاز به تجویز ضد‐درد بیشتر را کاهش دهد. عوارض جانبی بالقوه هم در زنان و هم در نوزادان به درستی ارزیابی نشد. کارآزماییهای دیگر باید به شکافهای موجود در شواهد مربوط به پیامدهای مادر مانند رضایت از مراقبتهای پس از زایمان، عملکرد/بهزیستی (well‐being) مادر (وابستگی عاطفی، خود‐کارآمدی، شایستگی، استقلال، اعتماد به نفس، مراقبت از خود، مهارتهای کنار آمدن با مسائل) و عوارض جانبی دارو در نوزادان بپردازند.
Contexte: Les douleurs périnéales sont un effet indésirable courant, mais très peu étudié, consécutif à un accouchement. La douleur pourrait résulter d'un traumatisme périnéal dû à des ecchymoses, des déchirures spontanées, des incisions chirurgicales (épisiotomies), ou en association avec des extractions instrumentales (accouchement assisté par ventouse ou forceps). Il s'agit d'une mise à jour d'une revue publiée pour la dernière fois en 2013.
Objectifs: Déterminer l'efficacité d'une seule administration de paracétamol (acétaminophène) utilisée pour soulager les douleurs périnéales aiguës du post‐partum. STRATÉGIE DE RECHERCHE DOCUMENTAIRE: Pour cette mise à jour, nous avons effectué des recherches dans le registre des essais du groupe Cochrane sur la grossesse et l’accouchement, sur le site ClinicalTrials.gov, sur le Système d'enregistrement international des essais cliniques (ICTRP) (9 décembre 2019) et sur les références bibliographiques des études récupérées. CRITÈRES DE SÉLECTION: Essais contrôlés randomisés (ECR), y compris les ECR en grappes, comparant le paracétamol au placebo. Nous avons exclu les quasi‐ECR et les essais croisés. Les données provenant des résumés ne seront incluses que si les auteurs ont confirmé par écrit que les données à inclure dans la revue proviennent de l'analyse finale et ne seront pas modifiées. RECUEIL ET ANALYSE DES DONNÉES: Deux auteurs de la revue ont évalué chaque étude pour l'inclusion et l'extraction des données. Un auteur de la revue a examiné les décisions et confirmé les calculs des scores de soulagement de la douleur. Nous avons évalué le niveau de confiance des données probantes en utilisant la méthode GRADE. RÉSULTATS PRINCIPAUX: Cette mise à jour n'a pas identifié de nouvel essai, de sorte que les résultats restent inchangés. Toutefois, en appliquant l'évaluation des données probantes par la méthode GRADE, l'interprétation des principaux résultats a différé de la version précédente de cette revue. Nous avons identifié 10 études portant sur 2044 femmes, mais toutes ces études ont porté sur trois ou quatre groupes, et ont porté sur des médicaments ou des doses différentes. Nous n'avons inclus que les 1301 femmes qui se trouvaient dans les bras paracétamol comparé au placebo des études. Parmi celles‐ci, cinq études (482 femmes) ont évalué 500 mg à 650 mg et six études (797 femmes) ont évalué 1000 mg de paracétamol. Une étude a évalué 650 mg et 1000 mg par rapport à un placebo et a contribué aux deux comparaisons. Nous avons utilisé une méta‐analyse à effets aléatoires en raison de la variabilité clinique entre les études. Ces études dataient des années 70 et s'étendaient jusqu'au début des années 90. Elles contenaient des informations insuffisantes pour évaluer correctement les risques de biais. Les conclusions doivent donc être interprétées selon ce contexte précis. Le niveau de confiance des données probantes pour les deux critères de jugement principaux sur lesquels des données étaient disponibles a été évaluée comme faible, abaissé en raison d’un risque global de biais peu clair et de l'hétérogénéité (statistique I² de 60 % ou plus). Davantage de femmes pourraient ressentir un soulagement de la douleur avec le paracétamol par rapport au placebo (risque relatif (RR) moyen 2,14, intervalle de confiance (IC) à 95 % 1,59 à 2,89 ; 10 essais, 1279 femmes), et moins de femmes pourraient avoir besoin d'un soulagement supplémentaire de la douleur avec le paracétamol par rapport au placebo (RR moyen 0,34, IC à 95 % 0,21 à 0,55 ; 8 essais, 1132 femmes). Cependant, le niveau de confiance des données probantes était faible, abaissée en raison d'un risque global de biais peu clair et d'une hétérogénéité substantielle. Une étude a utilisé la dose la plus élevée de paracétamol (1000 mg) et rapporté les effets indésirables des médicaments chez la mère. Il pourrait y avoir peu ou pas de différence dans l'incidence de la nausée (RR moyen 0,18, IC à 95 % 0,01 à 3,66 ; 1 essai, 232 femmes ; données probantes d’un niveau de confiance faible), ou de la somnolence (RR moyen 0,89, IC à 95 % 0,18 à 4,30 ; 1 essai, 232 femmes ; données probantes d’un niveau de confiance faible). Aucun autre événement indésirable chez la mère n'a été rapporté. Aucune de ces études n'a évalué les effets indésirables des médicaments chez les nouveau‐nés.
Conclusions des auteurs: Une dose unique de paracétamol pourrait améliorer le soulagement de la douleur périnéale après un accouchement par voie basse, et pourrait réduire la nécessité d'un soulagement supplémentaire de la douleur. Les effets indésirables potentiels pour les femmes et les nouveau‐nés n'ont pas été évalués de manière appropriée. Tout nouvel essai devrait également aborder les lacunes dans les données probantes concernant les critères de jugement maternels tels que la satisfaction des soins postnatals, le fonctionnement/bien‐être maternel (attachement émotionnel, auto‐efficacité, compétence, autonomie, confiance, soins personnels, capacités d'adaptation) et les effets indésirables des médicaments chez les nouveau‐nés.
Antecedentes: El dolor perineal es un desenlace adverso frecuente, pero poco estudiado, que se presenta después del parto. El dolor puede ser resultado del traumatismo perineal debido a hematomas, desgarros espontáneos, incisiones quirúrgicas (episiotomías), o estar asociado con procedimientos quirúrgicos durante el parto (ventosa o fórceps para ayudar al parto). Ésta es una actualización de una revisión publicada en 2013.
Objetivos: Determinar la eficacia de una administración única de paracetamol (acetaminofeno) para aliviar el dolor perineal posparto agudo. MÉTODOS DE BÚSQUEDA: Para esta actualización se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Embarazo y parto (Cochrane Pregnancy and Childbirth), ClinicalTrials.gov, la Plataforma de registros internacionales de ensayos clínicos de la OMS (9 de diciembre de 2019, y en las listas de referencias de los estudios obtenidos. CRITERIOS DE SELECCIÓN: Ensayos controlados aleatorizados (ECA), incluidos los ECA por conglomerados, que compararon el paracetamol con placebo. Se excluyeron los ensayos cruzados (cross‐over) y los ensayos cuasialeatorizados. Los datos de los resúmenes solo se incluirían si los autores hubieran confirmado por escrito que los datos que se iban a incluir en la revisión procedían del análisis final y no cambiarían. OBTENCIÓN Y ANÁLISIS DE LOS DATOS: Dos autores de la revisión evaluaron cada artículo para su inclusión y extrajeron los datos. Un autor de la revisión examinó las decisiones y confirmó los cálculos de las puntuaciones de alivio del dolor. La certeza de la evidencia se evaluó mediante los criterios GRADE.
Resultados principales: En esta actualización no se identificaron nuevos ensayos, por lo que los resultados se mantienen sin cambios. Sin embargo, al aplicar la evaluación GRADE de la evidencia, la interpretación de los resultados principales difirió de la versión anterior de esta revisión. Se identificaron diez estudios con 2044 mujeres, pero todos estos estudios incluyeron tres o cuatro grupos que analizaron diferentes fármacos o dosis. Solo se han incluido a las 1301 mujeres que estaban en los brazos de paracetamol versus placebo de los estudios. De estos estudios, cinco (482 mujeres) evaluaron 500 mg a 650 mg y seis (797 mujeres) evaluaron 1000 mg de paracetamol. Un estudio evaluó 650 mg y 1000 mg en comparación con placebo y contribuyó a ambas comparaciones. Debido a la variabilidad clínica entre los estudios se utilizó un metanálisis de efectos aleatorios. Los estudios se realizaron desde los años setenta hasta principios de los años noventa y no hubo información suficiente para evaluar adecuadamente el riesgo de sesgo, por lo que los resultados se deben interpretar en ese contexto. La certeza de la evidencia de los dos desenlaces principales sobre los que hubo datos disponibles se consideró baja, y se disminuyó por riesgo general de sesgo incierto y por la heterogeneidad (estadística I² del 60% o mayor). Un mayor número de mujeres puede experimentar alivio del dolor con el paracetamol en comparación con placebo (razón de riesgos [RR] promedio 2,14; intervalo de confianza [IC] del 95%: 1,59 a 2,89; diez ensayos, 1279 mujeres), y un menor número de mujeres puede necesitar un alivio adicional del dolor con el paracetamol en comparación con placebo (RR promedio 0,34; IC del 95%: 0,21 a 0,55; ocho ensayos, 1132 mujeres). Sin embargo, la certeza de la evidencia fue baja, y se disminuyó debido al riesgo general de sesgo y a la heterogeneidad significativa. Un estudio utilizó la dosis más alta de paracetamol (1000 mg) e informó sobre los efectos adversos del fármaco en la madre. Puede haber poca o ninguna diferencia en la incidencia de náuseas (RR promedio 0,18; IC del 95%: 0,01 a 3,66; un ensayo, 232 mujeres; evidencia de certeza baja), o la somnolencia (RR promedio 0,89; IC del 95%: 0,18 a 4,30; un ensayo, 232 mujeres; evidencia de certeza baja). No se informaron otros eventos adversos en la madre. Ninguno de los estudios evaluó los efectos adversos de los fármacos en los neonatos.
Conclusiones de los autores: Una sola dosis de paracetamol puede mejorar el alivio del dolor perineal después de un parto vaginal y puede reducir la necesidad de un alivio adicional del dolor. No se evaluaron adecuadamente los posibles efectos adversos en las mujeres ni en los neonatos. Cualquier ensayo adicional debe abordar las insuficiencias en la evidencia relacionadas con desenlaces maternos como la satisfacción con la atención posnatal, la funcionalidad/bienestar materno (apego emocional, autoeficacia, competencia, autonomía, confianza, autocuidado, habilidades de afrontamiento) y los efectos adversos de los fármacos en los neonatos.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
EA: none.
YS: none.
GG: has received royalties from John Wiley & Sons in respect of "
Figures
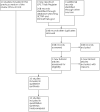







Update of
-
Paracetamol/acetaminophen (single administration) for perineal pain in the early postpartum period.Cochrane Database Syst Rev. 2013 Jan 31;(1):CD008407. doi: 10.1002/14651858.CD008407.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2021 Jan 8;1:CD008407. doi: 10.1002/14651858.CD008407.pub3. PMID: 23440827 Updated.
Comment in
-
Single-Dose Oral NSAIDs and Acetaminophen for Perineal Pain in the Early Postpartum Period.Am Fam Physician. 2021 Dec 1;104(6):575-577. Am Fam Physician. 2021. PMID: 34913657 No abstract available.
References
References to studies included in this review
Behotas 1992 {published data only}
-
- Behotas S, Chauvin A, Castiel J, Martin A, Boureau F, Barrat J, et al. Analgesic effect of ibuprofen in pain after episiotomy [Effets antalgiques de l'ibuprofene dans les douleurs apres episiotomie]. Annales Francaises d'Anesthesie et de Reanimation 1992;11(1):22-6. - PubMed
Hopkinson 1973 {published data only}
-
- Hopkinson JH, Bartlett FH, Steffens AO, McGlumphy TH, Macht EL, Smith M. Acetaminophen vs propoxyphene hydrochloride for relief of pain in episiotomy patients. Journal of Clinical Pharmacology 1973;13:251-63. - PubMed
Hopkinson 1974 {published data only}
-
- Hopkinson IJ, Smith MT, Bare WW. Acetaminophen (500 mg.) versus acetaminophen (325 mg.) for the relief of pain in episiotomy patients. Current Therapeutic Research 1974;16(3):194-200.
Hopkinson 1976 {published data only}
-
- Hopkinson JH, Blatt G, Cooper M, Levin HM, Berry FN, Cohn H. Effective pain relief: comparative results with acetaminophen in a new dose formulation, propoxyphene napsylate acetaminophen combination, and placebo. Current Therapeutic Research, Clinical and Experimental 1976;19:622-30. - PubMed
Levin 1974 {published data only}
-
- Levin HM, Bare WW, Berry FN, Miller JM. Acetaminophen with codeine for the relief of severe pain in postpartum patients. Current Therapeutic Research, Clinical and Experimental 1974;16(9):921-7. - PubMed
Melzack 1983 {published data only}
-
- Melzack R, Jeans ME, Kinch RA, Katz J. Diflunisal (1000 mg single dose) vs acetaminophen (650 mg) and placebo for the relief of post-episiotomy pain. Current Therapeutic Research 1983;34:929-39.
Rubin 1984 {published data only}
-
- Rubin A, Winter L Jr. A double-blind randomized study of an aspirin/caffeine combination versus acetaminophen/aspirin combination versus acetaminophen versus placebo in patients with moderate to severe post-partum pain. Journal of International Medical Research 1984;12:338-45. - PubMed
Schachtel 1989 {published data only}
-
- Schachtel BP, Thoden WR, Baybutt RI. Ibuprofen and acetaminophen in the relief of postpartum episiotomy pain. Journal of Clinical Pharmacology 1989;29:550-3. - PubMed
-
- Schachtel BP, Thoden WR. Efficacy of ibuprofen 400mg, acetaminophen 1000mg and placebo in post-episiotomy pain. Clinical Pharmacology and Therapeutics 1989;45:175.
Smith 1975 {published data only}
-
- Smith MT, Levin HM, Bare WW, Berry FN, Miller JM. Acetaminophen extra strength capsules versus propoxyphene compound-65 versus placebo: a double-blind study of effectiveness and safety. Current Therapeutic Research, Clinical and Experimental 1975;17:452-9. - PubMed
Sunshine 1989a {published data only}
-
- Sunshine A, Zighelboim I, De Castro A, Sorrentino JV, Smith DS, Bartizek RD, et al. Augmentation of acetaminophen analgesia by the antihistamine phenyltoloxamine. Journal of Clinical Pharmacology 1989;29:660-4. - PubMed
References to studies excluded from this review
Abboud 1994 {published data only}
-
- Abboud TK, Zhu J, Longhitano M, Minehart M, Mantilla M, Chu G, et al. Efficacy and safety of butorphanol nasal spray for the relief of postepisiotomy pain. Current Therapeutic Research, Clinical and Experimental 1994;55(5):500-9.
Aissaoui 2008 {published data only}
-
- Aissaoui Y, Bruyere R, Mustapha H, Bry D, Kamili ND, Miller C. A randomized controlled trial of pudendal nerve block for pain relief after episiotomy. Anesthesia & Analgesia 2008;107(2):625-9. - PubMed
Azpiroz 1971 {published data only}
-
- Azpiroz P, Garcia G. Clinical trial of a new analgesic CI-473 Parke Davis, in puerperal pain [Ensayo clinico de un nuevo anagesico CI-473 Parke Davis, en los entuertos puerperales]. Tokoginecologia Practica 1971;30:135-59.
Beaver 1980 {published data only}
Benson 1963 {published data only}
-
- Benson RC. Double blind evaluation of analgesic agents in the postpartum patient. Western Journal of Surgery 1963;71:167-9. - PubMed
Bettigole 1981 {published data only}
-
- Bettigole JB. A double-blind comparison of placebo, codeine, and fenoprofen in patients with postpartum pain. Current Therapeutic Research 1981;29:778-84.
Bhounsule 1990 {published data only}
-
- Bhounsule SA, Nevreker PR, Agshikar NV, Pal MN, Dhume VG. A comparison of four analgesics in post-episiotomy pain. Indian Journal of Physiology and Pharmacology 1990;34(1):34-8. - PubMed
Bloomfield 1967 {published data only}
-
- Bloomfield SS, Gaffney TE, Howett M. Comparative analgesic efficacy of chlorphenesin carbamate and acetylsalicylic acid after episiotomy. Anesthesia and Analgesia 1967;46:515-20. - PubMed
Bloomfield 1970a {published data only}
-
- Bloomfield SS, Hurwitz HN. Tourniquet and episiotomy pain as test models for aspirin-like analgesics. Journal of Clinical Pharmacology 1970;10:361-9. - PubMed
Bloomfield 1970b {published data only}
-
- Bloomfield SS, Barden TP, Hille R. Clinical evaluation of flufenisal, a long-acting analgesic. Clinical Pharmacology and Therapeutics 1970;11:747-54. - PubMed
Bloomfield 1974 {published data only}
-
- Bloomfield SS, Barden TP, Mitchell J. Comparative efficacy of ibuprofen and aspirin in episiotomy pain. Clinical Pharmacology and Therapeutics 1974;15:565-70. - PubMed
Bloomfield 1980 {published data only}
Bloomfield 1981 {published data only}
-
- Bloomfield SS, Barden TP, Mitchell J. Propiram and codeine in episiotomy pain. International Journal of Clinical Pharmacology, Therapeutics and Toxicology 1981;19:152-7. - PubMed
-
- Bloomfield SS, Barden TP, Mitchell R. Propiram, codeine, pentazocine and propoxyphene in episiotomy pain. Clinical Pharmacology & Therapeutics 1979;25(2):215.
Bloomfield 1983 {published data only}
-
- Bloomfield SS, Sinkfield A, Mitchell J, Bichlmeir G, Barden TP. Ciramadol (Wy-15,705) and codeine analgesia after episiotomy. In: National Institute of Drug Abuse Research Monograph Series 43. Washington (DC): United States Department of Health and Human Services, 1983:224-30. - PubMed
Bloomfield 1985 {published data only}
-
- Bloomfield SS, Nelson ED, Mitchell J, Peters N, Cissell G, Barden TP. Flupirtine and acetaminophen analgesia after episiotomy. Clinical Pharmacology and Therapeutics 1985;37:182.
Bloomfield 1988a {published data only}
-
- Bloomfield SS. The comparative efficacy of Voltaren(R) (diclofenac), naproxen sodium and placebo in the treatment of postepisiotomy pain [Personal communication]. Conversation with: SS Bloomfield 20 April 1990.
Bloomfield 1988b {published data only}
-
- Bloomfield SS. Comparisons of the safety and efficacy of single doses of flupirtine maleate, acetaminophen (APAP), flupirtine plus APAP, and placebo in the treatment of postepisiotomy pain [Personal communication]. Conversation with: SS Bloomfield. 20 April 1990.
Bruni 1965 {published data only}
-
- Bruni JR, Holt RE. Controlled double-blind evaluation of three analgesic medications for postpartum discomfort. Obstetrics & Gynecology 1965;25:76-81. - PubMed
Bucheli 1994 {published data only}
-
- Bucheli R, Davalos V, Neto N, Naranjo I, Calderon D, Alamo C, et al. A randomized, double-blind study of the efficacy and safety of microcapsulated butibufen and naproxen in the treatment of post-episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1994;55(12):1527-37.
Buck 1978 {published data only}
-
- Buck ME, Paintin DB. Diflunisal in post-episiotomy pain: a preliminary report of a double-blind comparative study. Current Medical Research and Opinion 1978;5:548-9. - PubMed
Cater 1985 {published data only}
-
- Cater M, O'Brien PM, Pickvance NJ. A double-blind comparison of the new ibuprofen-codeine phosphate combination, zomepirac, and placebo in the relief of postepisiotomy pain. Clinical Therapeutics 1985;7:442-7. - PubMed
Choi 2000 {published data only}
-
- Choi DM, Peter EA, Douglas MJ, Janssen P. Naproxen and epidural morphine for perineal pain after forceps delivery. Anesthesiology 2000;92 Suppl:A79.
Churchill 1995 {published data only}
-
- Churchill D, Buxton EJ, Mann M, Luesley DM. Bupivacaine with adrenaline infiltration following episiotomy repair. Journal of Obstetrics and Gynaecology 1995;15:29-30.
Coburn 1966 {published data only}
-
- Coburn WA, Rutherford RN, Banks AL. Short-term use of oxyphenbutazone in the postpartum period. Obstetrics & Gynecology 1966;28:484-90. - PubMed
Colacioppo 2009 {published data only}
-
- Colacioppo PM, Gonzalez Riesco ML. Effectiveness of local anaesthetics with and without vasoconstrictors for perineal repair during spontaneous delivery: double-blind randomised controlled trial. Midwifery 2009;25(1):88-95. - PubMed
Daftary 1980 {published data only}
-
- Daftary SN, Mehta AC, Nanavati M. A controlled comparison of dipyrone and paracetamol in post-episiotomy pain. Current Medical Research and Opinion 1980;6:614-8. - PubMed
Defoort 1983 {published data only}
-
- Defoort P, Thiery M, Martens G, Van Maele G. A double-blind trial of single-dose ciramadol for the treatment of post-episiotomy pain. Current Medical Research and Opinion 1983;8:481-6. - PubMed
De los Santos 1998 {published data only}
-
- De los Santos AR, Marti MI, Espinosa D, Di Girolamo G, Vinacur JC, Casadei A. Lysine clonixinate vs paracetamol/codeine in postepisiotomy pain. Acta Physiologica, Pharmacologica et Therapeutica Latinoamericana 1998;48:52-8. - PubMed
De Vroey 1978 {published data only}
-
- De Vroey P. A double-blind comparison of diflunisal and aspirin in the treatment of post-operative pain after episiotomy. Current Medical Research and Opinion 1978;5:544-7. - PubMed
-
- De Vroey P. The treatment of postoperative pain with a single dose of diflunisal. Clinical Therapeutics 1977;1(Suppl A):30-3.
Farzin 1969 {published data only}
-
- Farzin B. A clinical evaluation of anti-inflammatory drugs in episiotomy: double-blind study comparing tanderil (oxyphenobutazone), and enzyme preparation (ananase) and placebo. South African Medical Journal 1969;43:1296.
Finch 1971 {published data only}
-
- Finch JS, DeKornfeld TJ. Clonixin: a clinical evaluation of a new oral analgesic. Journal of Clinical Pharmacology & New Drugs 1971;11(5):371-7. - PubMed
Fragen 1982 {published data only}
-
- Fragen RJ, Vlasuk L. Analgesic efficacy of a combination of fenoprofen and codeine administered orally. Current Therapeutic Research, Clinical and Experimental 1982;31(2):129-37.
Friedrich 1983 {published data only}
-
- Friedrich E. A comparison of etodolac (Ultradol) with aspirin and placebo in patients with episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1983;33(1):100-7.
Fyneface‐Ogan 2006 {published data only}
-
- Fyneface-Ogan S, Mato CN, Enyindah CE. Postpartum perineal pain in primiparous women: a comparison of two local anaesthetic agents. Nigerian Journal of Medicine 2006;15(1):77-80. - PubMed
Gindhart 1971 {published data only}
-
- Gindhart JD. A rationale for studying analgesia. A double blind study in postpartum patients. Current Therapeutic Research 1971;13:240-50. - PubMed
Gleason 1987 {published data only}
-
- Gleason JA, Winer W, Turner JL. Comparison of meclofenamate sodium with codeine and placebo for the treatment of episiotomy pain. Clinical Therapeutics 1987;9(6):585-93. - PubMed
Gruber 1962 {published data only}
-
- Gruber CM, Baptisti A, Chernish SM. Comparative evaluation of analgesic agents in postpartum patients: oral dextropropoxyphene, codeine and meperidine. Anesthesia and Analgesia 1962;41:538-44. - PubMed
Gruber 1976 {published data only}
-
- Gruber CM. Evaluating interactions between fenoprofen and propoxyphene: analgesia and adverse reports by postepisiotomy patients. Journal of Clinical Pharmacology 1976;16(8-9):407-17. - PubMed
Gruber 1977 {published data only}
-
- Gruber CM. Codeine and propoxyphene in postepisiotomy pain. A two-dose evaluation. Journal of the American Medical Association 1977;237:2734-5. - PubMed
Gruber 1979 {published data only}
-
- Gruber CM, Bauer RO, Bettigole JB, Lash AF, McDonald JS. A multicenter study for analgesia involving fenoprofen, propoxyphene [alone or in combination] with placebo and aspirin controls in postpartum pain. Journal of Medicine 1979;10(1-2):65-98. - PubMed
Harrison 1987 {published data only}
-
- Harrison RF, Brennan M. Comparison of two formulations of lignocaine spray with mefenamic acid in the relief of post-episiotomy pain: a placebo-controlled study. Current Medical Research and Opinion 1987;10:375-9. - PubMed
Harrison 1992 {published data only}
-
- Harrison RF, Devitt M. Indomethacin and ethamsylate alone and in combination for the relief of post episiotomy pain. Irish Journal of Medical Science 1992;161(8):493-7. - PubMed
Hebertson 1986 {published data only}
-
- Hebertson RM, Storey N, Turner JL. Analgesic efficacy of meclofenamate sodium in episiotomy pain. Pharmacotherapy 1986;6:205-10. - PubMed
Hofmeyr 1990 {published data only}
-
- Hofmeyr GJ, Piccioni V, Blauhof P. Postpartum homoeopathic Arnica montana: a potency-finding pilot study. British Journal of Clinical Practice 1990;44:619-21. - PubMed
Honorato 1990 {published data only}
-
- Honorato J, Caballero R, Giorgiani G, Movilia PG, Tapounet R. Dose-analgesic response study and aceclofenac plasma levels in humans. Current Therapeutic Research, Clinical and Experimental 1990;47(4):605-11.
Hopkinson 1978 {published data only}
-
- Hopkinson JH. Hydrocodone. A unique challenge for an established drug: comparison of repeated oral doses of hydrocodone (10 mg) and codeine (60 mg) in the treatment of postpartum pain. Current Therapeutic Research, Clinical and Experimental 1978;24(5):503-16.
Hopkinson 1980 {published data only}
-
- Hopkinson JH. Ibuprofen vs propoxyphene hydrochloride and placebo in the relief of postepisiotomy pain. Current Therapeutic Research 1980;27:55-63.
Jacobson 1987 {published data only}
-
- Jacobson J, Bertilson SO. Analgesic efficacy of paracetamol/codeine and paracetamol/dextropropoxyphene in pain after episiotomy and ruptures in connection with childbirth. Journal of International Medical Research 1987;15:89-95. - PubMed
Jain 1978a {published data only}
-
- Jain AK, McMahon FG, Ryan JR, Unger D, Richard W. A comparison of aspirin-caffeine versus aspirin: results of two double-blind placebo controlled studies in postpartum pain [abstract]. Clinical Pharmacology and Therapeutics 1978;23(1):116. - PubMed
-
- Jain AK, McMahon FG, Ryan JR, Unger D, Richard W. Aspirin and aspirin-caffeine in postpartum pain relief. Clinical Pharmacology and Therapeutics 1978;24:69-75. - PubMed
Jain 1978b {published data only}
-
- Jain AK, McMahon FG, Ryan JR, Raphan H, Richard W. Piroxicam, a novel analgesic in postpartum pain. European Journal of Rheumatology and Inflammation 1978;1(3):356-9.
Jain 1985 {published data only}
-
- Jain AK, McMahon FG, Ryan JR, Smith GB. Analgesic efficacy of indoprofen in postpartum episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1985;38(5):677-81.
Jain 1988 {published data only}
-
- Jain AK, Mcmahon FG, Ryan JR, Narcisse C. A double-blind study of ibuprofen 200 mg in combination with caffeine 100 mg, ibuprofen 400 mg, and placebo in episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1988;43(4):762-9.
Kamondetdecha 2008 {published data only}
-
- Kamondetdecha R, Tannirandorn Y. Ibuprofen versus acetaminophen for the relief of perineal pain after childbirth: a randomized controlled trial. Journal of the Medical Association of Thailand 2008;91(3):282-6. - PubMed
Kantor 1984 {published data only}
-
- Kantor T, Cavaliere MB, Hopper M, Roepke S. A double-blind parallel comparison of ketoprofen, codeine, and placebo in patients with moderate to severe postpartum pain. Journal of Clinical Pharmacology 1984;24:228-34. - PubMed
Khan 1987 {published data only}
-
- Khan GQ, Lilford RJ. Wound pain may be reduced by prior infiltration of the episiotomy site after delivery under epidural analgesia. British Journal of Obstetrics and Gynaecology 1987;94:341-4. - PubMed
Lasagna 1967 {published data only}
-
- Lasagna L, Davis M, Pearson JW. A comparison of acetophenetidin and acetaminophen. I Analgesic effects in postpartum patients. Journal of Pharmacology and Experimental Therapeutics 1967;155:296-300. - PubMed
Laska 1981 {published data only}
-
- Laska EM, Sunshine A. Fenoprofen and codeine analgesia. Clinical Pharmacology and Therapeutics 1981;29:606-16. - PubMed
Laska 1983 {published data only}
-
- Laska EM, Sunshine A, Zighelboim I, Roure C, Marrero I, Wanderling J, et al. Effect of caffeine on acetaminophen analgesia. Clinical Pharmacology and Therapeutics 1983;33(4):498-509. - PubMed
Laska 1984 {published data only}
-
- Laska EM, Sunshine A, Mueller F, Elvers WB, Siegel C, Rubin A. Caffeine as an analgesic adjuvant. Journal of the American Medical Association 1984;251:1711-33. - PubMed
Lataste 1981 {published data only}
-
- Lataste X, Berchier P. Clinical evaluation of fluproquazone in post-operative pain. A report of double-blind comparative trials in patients after surgical interventions. Arzneimittel-Forschung 1981;31(5a):920-4. - PubMed
Lett 2007 {published data only}
-
- Lett C. Oral versus rectal administration of naproxen for post-vaginal delivery of perineal pain control: a randomized trial. Journal of Obstetrics and Gynaecology Canada 2007;29(6 Suppl 1):S15.
Levin 1978a {published data only}
-
- Levin HM. Relative potency assay comparing zomepirac sodium, propoxyphene napsylate, propoxyphene napsylate with acetaminophen, codeine, and placebo in patients with episiotomy pain. International Journal of Clinical Pharmacology and Therapeutics 1978;23(1):118.
Levin 1978b {published data only}
-
- Levin HM, Sanzari NP, Losada M, Caruso FS. Double-blind oral analgesic study of butorphanol in episiotomy pain: a comparison with codeine and placebo. Journal of International Medical Research 1978;6:24-33. - PubMed
Levin 1979 {published data only}
-
- Levin HM, Schlein AN, Sanzari NP. Efficacy of viminol in episiotomy pain. Clinical Pharmacology and Therapeutics 1979;25(2):234.
Lim 2008 {published data only}
-
- Lim SS, Tan PC, Sockalingam JK, Omar SZ. Oral celecoxib versus oral diclofenac for post-perineal repair analgesia after spontaneous vaginal birth: a randomised trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2008;48(1):71-7. - PubMed
London 1983a {published data only}
-
- London RS, Sundaram GS, Feldman S, Goldstein PJ. Aspirin in the treatment of episiotomy pain. Southern Medical Journal 1983;76:844-5. - PubMed
London 1983b {published data only}
-
- London R, Sundaram GS, Feldman S, Goldstein PJ. Episiotomy pain: efficacy and safety of fluproquazone compared to aspirin and placebo. International Journal of Gynecology & Obstetrics 1983;21:251-5. - PubMed
Mazzarella 1989 {published data only}
-
- Mazzarella B, Mastronardi P, Rossi AE, Cafiero T, Chiefari M, Ceccarelli G. Controlled clinical evaluation of the analgesic efficacy of flupirtine and ketoprofen in postepisiotomy pain. In: 4th European Congress of Allied Specialists in Maternal and Neonatal Care; 1989 Sept 12–15; Bruges, Belgium. 1989.
McCallum 1991 {published data only}
-
- McCallum KA. Trial to assess Ponstan vs placebo, given within an hour of birth, to women who had had epidural and forceps deliveries, as a means of prophylactic analgesia, by measuring time to first postnatal analgesic consumption [Personal communication]. Conversation with KA McCallum 1991.
Moggian 1972 {published data only}
-
- Moggian G, Cervellati I, Tamburini E. Critical study of the post-partum pain by means of a clinical pharmacology experiment. Bollettino Chimico Farmaceutico 1972;111(8):497-9. - PubMed
Movilia 1989 {published data only}
-
- Movilia PG. Evaluation of the analgesic activity and tolerability of aceclofenac in the treatment of post-episiotomy pain. Drugs under Experimental and Clinical Research 1989;15(1):47-51. - PubMed
Mukherjee 1980 {published data only}
-
- Mukherjee S, Sood S. A controlled evaluation of orally administered aspirin dipyrone and placebo in patients with post-operative pain. Current Medical Research and Opinion 1980;6:619-23. - PubMed
Norman 1985 {published data only}
-
- Norman SL, Jeavons BI, O'Brien PM, Johnson IR, Hitchcock A, Noyelle RM, et al. A double-blind comparison of a new ibuprofen-codeine phosphate combination, codeine phosphate, and placebo in the relief of postepisiotomy pain. Clinical Therapeutics 1985;7(5):549-54. - PubMed
Noveck 1983 {published data only}
-
- Noveck RJ, Jain AK, Ryan JR, McMahon FG. Double-blind, placebo-controlled, analgesic efficacy of orally administered butorphanol tartrate/acetaminophen combination. Clinical Pharmacology and Therapeutics 1983;33:198.
Odigie 1988 {published data only}
-
- Odigie EA. Effectiveness of indomethacin (Indocid) suppositories as post-episiotomy analgesia. International Journal of Gynecology & Obstetrics 1988;26:57-60. - PubMed
Offen 1985 {published data only}
-
- Offen WW, Gruber CM. Dose response to fenoprofen calcium using placebo and codeine as controls. Journal of Medicine 1985;16(4):439-52. - PubMed
Ogunbode 1987 {published data only}
-
- Ogunbode O. A comparative trial of piroxicam and paracetamol after episiotomy wound repair. Current Therapeutic Research, Clinical & Experimental 1987;41:89-94.
Okun 1982 {published data only}
-
- Okun R. Evaluation of the analgesic effect of fendosal in patients with postpartum uterine cramp or episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1982;32(1):65-73.
Olson 1984 {published data only}
-
- Olson N, Sunshine A, Roure C, Colon A, Laska EM, Santiago H, et al. Analgesic efficacy of suprofen, codeine and placebo. Pain 1984;2 Suppl:238.
Olson 1997 {published data only}
-
- Olson NZ, Sunshine A, Zighelboim I, DeCastro A. Onset and duration of analgesia of diclofenac potassium in the treatment of postepisiotomy pain. American Journal of Therapeutics 1997;4:239-46. - PubMed
Olson 1999 {published data only}
-
- Olson NZ, Sunshine A, Zighelboim I, Lange R. Analgesic efficacy of liquid ketoprofen compared to liquid dipyrone and placebo administered orally as drops in postepisiotomy pain. International Journal of Clinical Pharmacology & Therapeutics 1999;37(4):168-74. - PubMed
Pedersen 1982 {published data only}
-
- Pedersen KO, Kristensen OK, Gram-Hansen J. Venalot Depot in episiotomy. Doctors' Weekly 1982;144(2295):92-3. - PubMed
Pedronetto 1975 {published data only}
-
- Pedronetto S, Gorini F, Mandelli V, Fuccella LM. Double blind trial of the new analgesic and anti inflammatory drug indoprofen in post episiotomic pain. Journal of International Medical Research 1975;3:16-20.
Peter 2001 {published data only}
Petho 1981 {published data only}
-
- Petho A. Efficacy of benzopyrenes in posttraumatic inflammations: clinical double blind study in the postoperative treatment of episiotomy [Die wirksamkeit von benzopyronen bei der posttraumatischen entzundung. Eine klinische doppelblindstudie in der nachbehandlung von scheidendammnahten]. Arzneimittel Forschung 1981;31:1303-7. - PubMed
Pitton 1982 {published data only}
-
- Pitton MA, Vincenti E, Polato D, Tambuscio B, Salvia D. Double-blind study on diclofenac in pain relief after episiotomy [Studio clinico a doppio cieco con diclofenac sodico nel controllo del dolore post-operatorio in ostetricia. Ii. Episiotomia]. Acta Anaesthesiologica Italica 1982;33(4):717-20.
Radman 1961 {published data only}
-
- Radman HM, Campbell C, Coplan RS. Anti-inflammatory drugs in obstetrics and gynaecology. American Journal of Obstetrics and Gynecology 1961;81:344-9. - PubMed
Ray 1993 {published data only}
-
- Ray S, Swami A, Kadim M, Morgan B. Efficacy of diclofenac in a single prophylactic dose in post partum pain. International Journal of Obstetric Anesthesia 1993;2:58.
Santiago 1959 {published data only}
-
- Santiago FS, Danforth DN. Non-narcotic analgesia to simplify postpartum care. Obstetrics & Gynecology 1959;13:22-3. - PubMed
Schinkel 2008 {published data only}
-
- Schinkel N, Colbus L, Soltner C, Parot-Schinkel E, Granry JC. Effect of local anesthetic infiltration for episiotomy repair among patients with epidural analgesia. Anesthesiology 2008;109:A1117.
Searles 1998 {published data only}
-
- Searles JA, Pring DW. Effective analgesia following perineal injury during childbirth: a placebo controlled trial of prophylactic rectal diclofenac. British Journal of Obstetrics and Gynaecology 1998;105(6):627-31. - PubMed
-
- Searles JA, Pring DW. Improving perineal pain relief. In: 27th British Congress of Obstetrics and Gynaecology; 1995 July 4-7; Dublin, Ireland. 1995:Abstract no: 499.
Smith 1994 {published data only}
-
- Smith JJ, O'Connor TC, Cooney CM, Gardiner J. Wound infiltration with morphine – an evaluation of pain relief after episiotomy. Anesthesia and Analgesia 1994;78:S408.
Sunshine 1981 {published data only}
-
- Sunshine A, Laska E, Zighelboim I, Desenne J. A comparison of the analgesic responses of fenoprofen, codeine, and placebo in postpartum and postoperative pain. Current Therapeutic Research, Clinical and Experimental 1981;29(5):771-7.
Sunshine 1983a {published data only}
-
- Sunshine A, Olson NZ, Laska EM, Zighelboim I, De Castro A, De Sarrazin C. Ibuprofen, zomepirac, aspirin, and placebo in the relief of postepisiotomy pain. Clinical Pharmacology and Therapeutics 1983;34:254-8. - PubMed
Sunshine 1983b {published data only}
-
- Sunshine A, Zighelboim I, De Sarrazin C. A study of the analgesic efficacy of nalbuphine hydrochloride in patients with postpartum pain. Current Therapeutic Research, Clinical and Experimental 1983;33(1):108-14.
-
- Sunshine A, Zighelboim I, Laska E. Oral analgesic study of nalbuphine hydrochloride, codeine, and placebo in postpartum pain. Clinical Pharmacology & Therapeutics 1982;31(2):274.
Sunshine 1983c {published data only}
-
- Sunshine A, Olson NZ, Laska EM, Zighelboim I, De Castro A, De Sarrazin C. Analgesic effect of graded doses of flurbiprofen in post-episiotomy pain. Pharmacotherapy 1983;3(3):177-81. - PubMed
Sunshine 1986 {published data only}
-
- Sunshine A, Olson JZ, Siegel C, Laska EM. Oral analgesic study of ketoprofen, aspirin and placebo in post-partum pain. Clinical Pharmacology and Therapeutics 1983;33:154.
-
- Sunshine A, Zighelboim I, Laska E, Siegel C, Olson NZ, De Castro A. A double-blind, parallel comparison of ketoprofen, aspirin, and placebo in patients with postpartum pain. Journal of Clinical Pharmacology 1986;26(8):706-11. - PubMed
Sunshine 1987a {published data only}
-
- Sunshine A, Zighelboim I, Olson N, Laska E. Flurbiprofen, flurbiprofen dextrorotatory component (BTS 24332) and placebo in post-episiotomy pain. Clinical Pharmacology and Therapeutics 1987;41:162.
Sunshine 1987b {published data only}
-
- Sunshine A, Roure C, Olson N, Laska EM, Zorrilla C, Rivera J. Analgesic efficacy of two ibuprofen-codeine combinations for the treatment of postepisiotomy and postoperative pain. Clinical Pharmacology and Therapeutics 1987;42:374-80. - PubMed
-
- Sunshine A, Roure C, Zorrilla C, Laska EM, Olson N, Rivera J. The analgesic efficacy of ibuprofen, codeine and placebo. Clinical Pharmacology and Therapeutics 1985;37:232. - PubMed
Sunshine 1989b {published data only}
-
- Sunshine A, Laska E, Siegel C, Zighelboim I, De Castro A, Sorrentino J, et al. Analgesic adjuvancy of caffeine with ibuprofen in three different postpartum pain populations. Clinical Pharmacology and Therapeutics 1989;45:174.
Szabados 1986 {published data only}
-
- Szabados T. Acemetacine and phenylbutazone: double-blind study in pain and inflammations following episiotomy [Doppelblindprufung von acemetacin und phenylbutazon bei schmerzen und entzundungen nach episiotomie]. Medizinische Welt 1986;37:703-6.
Taina 1981 {published data only}
-
- Taina E. Ibuprofen vs placebo in the relief of post-episiotomy pain. Current Medical Research and Opinion 1981;7:423-8. - PubMed
Trop 1983 {published data only}
-
- Trop D, Nucci C, Elie R, Gareau J. Double-blind comparative evaluation of tiaprofenic acid (Surgam) versus acetylsalicylic acid (ASA) in relieving pain following episiotomy. Current Therapeutic Research, Clinical and Experimental 1983;34(2I):274-9.
Van Wering 1972 {published data only}
-
- Van Wering RF, Bleker OP. Oral analgesia in post-partum pain: a comparison of ibuprofen ('Brufen') and dextropropoxyphene. Current Medical Research and Opinion 1972;1:49-52. - PubMed
Veltmann 1980 {published data only}
-
- Veltmann W. Sympathomimetics in the postoperative management of episiotomies. Results of a double blind clinical trial. Therapie der Gegenwart 1980;119:912-7. - PubMed
Visanto 1980 {published data only}
-
- Visanto J, Dubois D, Coquelin JP. Comparative study between antrafenine and placebo in patients with pain due to episiotomy. European Journal of Clinical Investigation 1980;10:39; Abstract no: 229.
Von Pein 1974 {published data only}
-
- Von Pein W. Double blind study using benzydamine in the puerperium. Gynakologische Rundschau 1974;14:327-8. - PubMed
Walters 1985 {published data only}
-
- Walters BN, Smith VA, De Swiet M, Mustill TA. Pain relief after episiotomy – a comparative study of suprofen and dihydrocodeine. British Journal of Obstetrics and Gynaecology 1985;92:1160-3. - PubMed
-
- Walters BN, Smith VA, De Swiet M. Comparative trial of suprofen and dihydrocodeine in post-episiotomy pain. Pain 1984;2:236. - PubMed
Wisanto 1981 {published data only}
-
- Wisanto A, Caudron J, Dubois D, Narbonne G, Coquelin JP. A double-blind placebo-controlled single-dose study comparing antrafenine and placebo in patients with post-episiotomy pain. Current Therapeutic Research, Clinical and Experimental 1981;29:171-82.
Yonkeura 1987 {published data only}
-
- Yonkeura ML, Petrone S, Turner JL, Di Zerega GS. Double-blind comparison of meclofenamate sodium with codeine and placebo for the pain of episiotomy. Clinical Therapeutics 1987;9:578-84. - PubMed
Yoong 1997 {published data only}
-
- Yoong WC, Biervliet F, Nagrani R. The prophylactic use of diclofenac (Voltarol) suppositories in perineal pain after episiotomy. A random allocation double-blind study. Journal of Obstetrics & Gynaecology 1997;17(1):39-41. - PubMed
Yscla 1988 {published data only}
-
- Yscla A. Aceclofenac and paracetamol in episiotomal pain. Drugs under Experimental and Clinical Research 1988;7:491-4. - PubMed
Additional references
Aasheim 2007
Andrews 2008
-
- Andrews V, Thakar R, Sultan AH, Jones PW. Evaluation of postpartum perineal pain and dyspareunia – a prospective study. European Journal of Obstetrics & Gynecology and Reproductive Biology 2008;137:152-6. - PubMed
Beckmann 2006
Begg 1996
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin L, et al. Improving the quality of reporting of randomized trials. The CONSORT statement. Journal of the American Medical Association 1996;276(8):637-9. - PubMed
Briggs 2008
-
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: a Reference Guide to Fetal and Neonatal Risk. 8th edition. Philadelphia: Lippincott Williams & Wilkins, 2008.
Burke 2008
-
- Burke A, Smyth E, Fitzgerald G. Chapter 26. Analgesic-antipyretic and anti-inflammatory agents; pharmacotherapy of gout. In: Goodman & Gilman's The Pharmacologic Basis of Therapeutics. 11 edition. New York (NY): McGraw-Hill, 2008.
Carroli 1999
-
- Carroli G, Belilzan J. Episiotomy for childbirth. Cochrane Database of Systematic Reviews 1999, Issue 3. Art. No: CD000081. [DOI: 10.1002/14651858.CD000081] - DOI
Cluett 2002
Cooper 1991
-
- Cooper SA. Commentary: single-dose analgesic studies: the upside and downside of assay sensitivity. In: Max M, Portenov R, Laska E, editors(s). Advances in Pain Research and Therapy. Vol. 18. New York (NY): Raven Press, 1991:117-24.
Cunningham 2005
-
- Cunningham G. Maternal anatomy. In: Cunningham G, Leveno K, Bloom S, Hauth J, Gilstrap L, Wenstrom K, editors(s). Williams Obstetrics. 22nd edition. New York (NY): McGraw-Hill, 2005:21.
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-analysis in Context. London (UK): BMJ Books, 2001.
Drugs and Lactation Database (LactMed) 2006
-
- Drugs and Lactation Database (LactMed). Acetaminophen. www.ncbi.nlm.nih.gov/books/NBK501194/ (accessed prior to 15 December 2020).
East 2007
Egger 1997
Greenshields 1993
-
- Greenshields W, Hulme H, Oliver S. The Perineum in Childbirth: a Survey of Women's Experiences and Midwives' Practices. London (UK): National Childbirth Trust, 1993.
Gupta 2004
Harbord 2006
-
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25:3443-57. - PubMed
Hay‐Smith 1998
Hedayati 2003
Hedayati 2005
Higgins 2009
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019.
Kearney 2020
-
- Kearney L. Can breastfeeding mothers take paracetamol or combination paracetamol products? Trent Regional Medicines information Service & UK Drugs in Lactation Advisory Service Prepared by UK Medicines Information (UKMi) pharmacists for NHS healthcare professionals. 02 April 2020.
Kettle 1999
Kettle 2004
-
- Kettle C. The pelvic floor. In: Henderson C, Macdonald S, editors(s). Mayes' Midwifery: a Textbook for Midwives. 13 edition. Edinburgh (UK): Bailliere Tindall, 2004:476-91.
Kettle 2007
Lawrence 2009
Macarthur 2004
-
- Macarthur AJ, Macarthur C. Incidence, severity, and determinants of perineal pain after vaginal delivery: a prospective cohort study. American Journal of Obstetrics and Gynecology 2004;192:1199-204. - PubMed
McQuay 2007
Moore 1996
-
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66:229-37. - PubMed
Moore 1997a
-
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesia: verification from independent data. Pain 1997;69:127-30. - PubMed
Moore 1997b
-
- Moore A, Moore O, McQuary H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesia: use of pain intensity and visual analogue scales. Pain 1997;69:311-5. - PubMed
Review Manager 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Smith 2009
-
- Smith H. Potential analgesic mechanisms of acetaminophen. Pain Physician 2009;12:269-80. - PubMed
Toms 2008
Williams 2007
-
- Williams A, Herron-Marx S, Hicks C. The prevalence of enduring postnatal perineal morbidity and its relationship to perineal trauma. Midwifery 2007;23:392-403. - PubMed
References to other published versions of this review
Chou 2009
-
- Chou D, Abalos E, Gyte GML, Gülmezoglu AM. Drugs for perineal pain in the early postpartum period: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007734. [DOI: 10.1002/14651858.CD007734.pub2] - DOI
Chou 2010
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

