Cell activity modulation and its specific function maintenance by bioinspired electromechanical nanogenerator
- PMID: 34559554
- PMCID: PMC8462902
- DOI: 10.1126/sciadv.abh2350
Cell activity modulation and its specific function maintenance by bioinspired electromechanical nanogenerator
Abstract
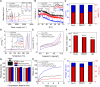
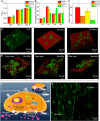
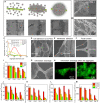
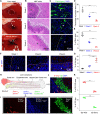
The biophysical characteristics of the extracellular matrix (ECM), such as a three-dimensional (3D) network and bioelectricity, have a profound influence on cell development, migration, function expression, etc. Here, inspired by these biophysical cues of ECM, we develop an electromechanical coupling bio-nanogenerator (bio-NG) composed of highly discrete piezoelectric fibers. It can generate surface piezopotential up to millivolts by cell inherent force and thus provide in situ electrical stimulation for the living cells. Besides, the unique 3D space in the bio-NGs provides an ECM-like growth microenvironment for cells. As a result, our bio-NGs effectively promote cell viability and development and, more importantly, maintain its specific functional expression. These advanced in vitro bio-NGs are expected to fill the gap between the inaccurate 2D systems and the expensive and time-consuming animal models, mimicking the complexity of the ECM and the physiological relevance of an in vivo biological system.
Figures



References
-
- Thrivikraman G., Boda S. K., Basu B., Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials 150, 60–86 (2018). - PubMed
-
- Ruan J. L., Tulloch N. L., Razumova M. V., Saiget M., Muskheli V., Pabon L., Reinecke H., Regnier M., Murry C. E., Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue. Circulation 134, 1557–1567 (2016). - PMC - PubMed
-
- Feng Z. Q., Wang T., Zhao B., Li J., Jin L., Soft graphene nanofibers designed for the acceleration of nerve growth and development. Adv. Mater. 27, 6462–6468 (2015). - PubMed
-
- Zheng Q., Tang Q., Wang Z. L., Li Z., Self-powered cardiovascular electronic devices and systems. Nat. Rev. Cardiol. 18, 7–21 (2021). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

