Instruments for assisted vaginal birth
- PMID: 34559884
- PMCID: PMC8462579
- DOI: 10.1002/14651858.CD005455.pub3
Instruments for assisted vaginal birth
Abstract
Background: Assisted vaginal births are carried out to expedite birth for the benefit of mothers and babies but are sometimes associated with significant morbidity for both. Various instruments are available, broadly divided into forceps and vacuum cups, and choice may be influenced by clinical circumstances, operator preference, experience and availability. OBJECTIVES: To evaluate the different instruments in terms of success in achieving a vaginal birth, and the risk of morbidity for mother and baby.
Search methods: We searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (14 May 2021), and reference lists of retrieved studies.
Selection criteria: We selected randomised controlled trials of assisted vaginal birth using different instruments. The review did not include quasi-randomised trials, cluster-randomised trials or cross-over designs. The review included trials for which abstracts alone were available as long as there was sufficient information to assess eligibility. DATA COLLECTION AND ANALYSIS: We used standard Cochrane methods. We used the GRADE approach to assess the certainty of evidence. The main outcomes assessed included failed delivery with allocated instrument, any maternal trauma, third- and fourth-degree tears, postpartum haemorrhage, any neonatal trauma, low Apgar and low umbilical artery pH. MAIN RESULTS: We included 31 studies involving a total of 5754 women. Risk of bias criteria were largely assessed as 'unclear', due to a lack of detail in trial reports. Blinding would have been challenging for all trials due to their inability to conceal the type of instrument used from either the woman or the operator, which is reflected in the risk of bias assessment. Any type of forceps versus any type of vacuum cup (12 studies, 3129 women) Forceps may be less likely to fail in achieving vaginal birth: risk ratio (RR) 0.58, 95% confidence interval (CI) 0.39 to 0.88; 11 studies, 3080 women; low certainty. 'Any maternal trauma' may be slightly more likely with forceps: odds ratio (OR) 1.53, 95% CI 0.98 to 2.40; 5 studies, 1356 women; low certainty; and third- or fourth-degree tears may also be more likely with forceps: RR 1.83, 95% CI 1.32 to 2.55; 9 studies, 2493 women; low certainty. There is no evidence of a difference in the incidence of postpartum haemorrhage (PPH) between the two groups: RR 1.71, 95% CI 0.59 to 4.95; 2 studies, 523 women; low certainty, because the evidence is very imprecise due to a very wide CI. More women in the forceps group reported requiring pain relief. There is probably no evidence of difference in rates of low Apgar: RR 0.83, 95% CI 0.46 to 1.51; 7 studies, 1644 women; moderate certainty; or low umbilical artery pH in the forceps group compared to any vacuum: RR 1.33, 95% CI 0.91 to 1.93; 2 studies, 789 women; low certainty; both of these outcomes are imprecise and have wide CIs that include both benefit and harm. There were also lower rates of fetal trauma with 'any forceps' (cephalhematoma, retinal haemorrhage and jaundice). The composite outcome of 'any neonatal trauma' was not reported. Low-cavity forceps versus any vacuum cup (2 studies, 218 women) We included two small studies with 218 participants in this comparison, but we judged most of the evidence as very low certainty, hence it was not feasible to make judgements on the difference in the rates of failed delivery, any maternal trauma or third- and fourth- degree tears. PPH and low umbilical artery pH were not reported. Soft vacuum cup versus any rigid cup (9 studies, 1148 women) Failed delivery may be more likely in the soft vacuum cup group: RR 1.62, 95% CI 1.21 to 2.17; 9 studies, 1148 women; low certainty. There may be no difference in the rates of 'any maternal trauma': OR 0.63, 95% CI 0.24 to 1.67; 2 studies, 348 women; low certainty, but the confidence interval is wide, indicating possible benefit or harm. There may be no difference in the rates of third- or fourth-degree tears: RR 0.93, 95% CI 0.35 to 2.44; 4 studies, 619 women; low certainty. There is probably no difference in the rates of PPH: RR 0.89, 95% CI 0.49 to 1.61; 5 studies, 737 women; moderate certainty between the soft and rigid cup groups. There may be little or no difference in the incidence of low Apgar scores: RR 0.82, 95% CI 0.49 to 1.37; 9 studies, 1148; low certainty; or low umbilical artery pH: RR 0.80, 95% CI 0.47 to 1.36; 1 study, 100 women; low certainty. Handheld vacuum versus any vacuum cup (4 studies, 968 women) There may be no difference in the rates of failures with allocated instrument: RR 1.35, 95% CI 0.81 to 2.25; 4 studies, 962 women; low certainty, any maternal trauma: OR 1.16, 95% CI 0.71 to 1.88; 2 studies; 394 women; low certainty, PPH: RR 0.31, 95% CI 0.03 to 2.92; 1 study, 164 women; low certainty, low umbilical artery pH: RR 1.06, 95% CI 0.71 to 1.59; 1 study, 164 women; low certainty, or low Apgar scores: RR 1.25, 95% CI 0.34 to 4.61; 3 studies, 784 women; low certainty) between the two groups. There is probably no difference in the rates of third- or fourth-degree tears between the 'handheld vacuum' and 'any vacuum cup' groups: RR 1.15, 95% CI 0.62 to 2.12; 4 studies, 962 women; moderate certainty.
Authors' conclusions: This review provides low-certainty evidence that forceps may be more likely to achieve vaginal birth and have lower rates of fetal trauma, but at a greater risk of perineal trauma and higher pain relief requirements compared with vacuum cups. There was low-certainty evidence that rigid vacuum cups may be more likely to achieve a vaginal birth than soft cups but with more fetal trauma, whilst handheld vacuum cups had similar success rates compared to other cups. There was no evidence of a difference in the rates of third- or fourth-degree tears or postpartum haemorrhages between types of cups, but wide confidence intervals around the estimates indicate further research is needed in this area.
Trial registration: ClinicalTrials.gov NCT01058200.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Ganga L Verma: none known.
Jessica J Spalding: none known.
Marc D Wilkinson: none known.
G Justus Hofmeyr: is author of one trial included in the study, and did not participate in decisions regarding this trial.
Valerie Vannevel: none known.
Fidelma O'Mahony: none known.
Figures


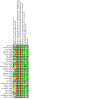
































































































































Update of
-
Choice of instruments for assisted vaginal delivery.Cochrane Database Syst Rev. 2010 Nov 10;(11):CD005455. doi: 10.1002/14651858.CD005455.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2021 Sep 24;9:CD005455. doi: 10.1002/14651858.CD005455.pub3. PMID: 21069686 Updated.
References
References to studies included in this review
Afifi 1995 {published data only}
-
- Afifi AW, Donia OT, Mampilly TP, El-Guindi MA. A randomized comparative study of the use of vacuum extraction with metal silastic cups in second stage management of deliveries in a Saudi Military Hospital. Saudi Medical Journal 1995;16(3):201-5.
Attilakos 2005 {published data only}
-
- Attilakos G, Sibanda T, Winter C, Johnson N, Draycott T. A randomised controlled trial of a new handheld vacuum extraction device. BJOG: an international journal of obstetrics and gynaecology 2005;112(11):1510-5. - PubMed
-
- Attilakos G, Sibanda T, Winter C, Johnson N, Draycott T. A randomised trial of a new handheld vacuum extraction device [abstract]. Journal of Obstetrics and Gynaecology 2004;24(Suppl 1):S23. - PubMed
Bofill 1996a {published data only}
-
- Bofill JA, Rust OA, Devidas M, Perry KG, Morrison JC, Martin JN Jr. Prognostic factors for the development of fetal cephalohematoma with vacuum extraction. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):316.
-
- Bofill JA, Rust OA, Devidas M, Roberts WE, Morrison JC, Martin JN Jr. Neonatal cephalohematoma from vacuum extraction. Journal of Reproductive Medicine 1997;42(9):565-9. - PubMed
-
- Bofill JA, Rust OA, Devidas M, Roberts WE, Morrison JC, Martin JN Jr. Shoulder dystocia and operative vaginal delivery. Journal of Maternal Fetal Medicine 1997;6(4):220-4. - PubMed
-
- Bofill JA, Rust OA, Schorr SJ, Brown RC, Martin RW, Martin JN Jr et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. American Journal of Obstetrics and Gynecology 1996;175(5):1325-30. - PubMed
-
- Bofill JA, Rust OA, Schorr SJ, Brown RC, Roberts WE, Morrison JC. A randomized trial of two vacuum extraction techniques. Obstetrics & Gynecology 1997;89(5 Pt 1):758-62. - PubMed
Carmody 1986 {published data only}
-
- Carmody F, Grant AM, Somchiwong M. Vacuum extraction: a randomized controlled comparison of the New Generation cup with the original Bird cup. Journal of Perinatal Medicine 1986;14(2):95-100. - PubMed
Chanwaro 1999 {published data only}
-
- Chanwaro Y, Ubolsa-ard S. Scalp injuries of metal and silastic cup vacuum extraction. Chon Buri Hospital Journal 1999;24(1):11-20.
Chenoy 1992 {published data only}
-
- Chenoy R, Johanson R. A randomized prospective study comparing delivery with metal and silicone rubber vacuum extractor cups. International Journal of Gynecology & Obstetrics 1993;40:189. - PubMed
-
- Chenoy R, Johanson RB. A randomized prospective study comparing delivery with metal and silicone rubber vacuum extractor cups. British Journal of Obstetrics and Gynaecology 1992;99(5):360-3. - PubMed
Cohn 1989 {published data only}
-
- Cohn M, Barclay C, Fraser R, Zaklama M, Johanson RB, Anderson D, et al. A multicentre randomized trial comparing delivery with a silicone rubber cup and rigid metal vacuum extractor cups. British Journal of Obstetrics and Gynaecology 1989;96(5):545-51. - PubMed
Dell 1985 {published data only}
-
- Dell DL, Sightler SE, Plauche WC. Soft cup vacuum extraction: a comparison of outlet delivery. Obstetrics & Gynecology 1985;66(5):624-8. - PubMed
Equy 2015 {published data only}
-
- David-Tschouda S. A randomized multicenter trial comparing vacuum assisted delivery with the new device "iCUP" versus the reference cup (ICUP). clinicaltrials.gov/ct2/show/NCT01058200 (first received 28 January 2010).
Fall 1986 {published data only}
-
- Fall O, Ryden G, Finnstrom K, Finnstrom O, Leijon I. Forceps or vacuum extraction? A comparison of effects on the newborn infant. Acta Obstetricia et Gynecologica Scandinavica 1986;65(1):75-80. - PubMed
Fitzpatrick 2003 {published data only}
-
- Fitzpatrick M, Behan M, O'Connell PR, O'Herlihy C. Randomised clinical trial to assess anal sphincter function following forceps or vacuum assisted vaginal delivery. BJOG: an international journal of obstetrics and gynaecology 2003;110(4):424-9. - PubMed
-
- Fitzpatrick M, Behan M, O'Connell PR, O'Herlihy C. Randomised comparison of anal sphincter function following forceps and vacuum delivery. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S160.
Groom 2006 {published data only}
-
- Groom KM, Jones BA, Miller N, Paterson-Brown S. A prospective randomised controlled trial of the Kiwi Omnicup versus conventional ventouse cups for vacuum-assisted vaginal delivery. BJOG: an international journal of obstetrics and gynaecology 2006;113(2):183-9. - PubMed
-
- Groom KM, Miller N, Jones BA, Paterson-Brown S. Randomized controlled prospective trial of the Kiwi Omnicup versus conventional vacuum device. Journal of Obstetrics and Gynaecology 2005;25:S24. - PubMed
Hammarström 1986 {published data only}
-
- Hammarström M, Csemiczky G, Belfrage P. Comparison between the conventional Malmström extractor and a new extractor with Silastic cup. Acta Obstetricia et Gynecologica Scandinavica 1986;65(7):791-2. - PubMed
Hebertson 1985 {published data only}
-
- Hebertson RM, Sanders MS, Warenski JC, Reed Heywood E, Larkin RM, Bryson MJ. Obstetric forceps pad designed to reduce infant trauma. Obstetrics & Gynecology 1985;65(2):275-8. - PubMed
Hofmeyr 1990 {published data only}
-
- Hofmeyr GJ, Gobetz L, Sonnendecker EW, Turner MJ. New design rigid and soft vacuum extractor cups: a preliminary comparison of traction forces. British Journal of Obstetrics and Gynaecology 1990;97(8):681-5. - PubMed
-
- Hofmeyr GJ, Gobetz L, Sonnendecker EW. A randomised comparison of traction forces and perinatal effects using various rigid and flexible vacuum extractor cups. In: Proceedings of Silver Jubilee British Congress of Obstetrics and Gynaecology; 1989 July 4-7; London, UK. 1989:191.
-
- Hofmeyr GJ, Gobetz L, Turner MJ. Perinatal effects of delivery with rigid and flexible vacuum extractor cups: a randomised study. In: 7th Conference on Priorities in Perinatal Care; 1988; South Africa. 1988:31-3.
Ismail 2008 {published data only}
-
- Ismail NA, Saharan WS, Zaleha MA, Jaafar R, Muhammad JA, Razi ZR. Kiwi omnicup versus malmström metal cup in vacuum assisted delivery: a randomized comparative trial. Journal of Obstetrics and Gynaecology Research 2008;34(3):350-3. - PubMed
Johanson 1989 {published data only}
-
- Johanson RB, Pusey J, Livera N, Jones P. North Staffordshire/Wigan assisted delivery trial. British Journal of Obstetrics and Gynaecology 1989;96(5):537-44. - PubMed
-
- Pusey J, Hodge C, Wilkinson P, Johanson RB. Maternal impressions of forceps or the Silc-cup. British Journal of Obstetrics and Gynaecology 1991;98(5):487-8. - PubMed
Johanson 1993 {published data only}
-
- Johanson R, Wilkinson P, Bastible A, Ryan S, Murphy H, O'Brien S. Health after childbirth: a comparison of normal and assisted vaginal delivery. Midwifery 1993;9(3):161-8. - PubMed
-
- Johanson RB, Heycock E, Carter J, Sultan AH, Walklate K, Jones PW. Maternal and child health after assisted vaginal delivery: five-year follow up of a randomised controlled study comparing forceps and ventouse. British Journal of Obstetrics and Gynaecology 1999;106(6):544-9. - PubMed
-
- Johanson RB, O'Brien PM, Rice C, Doyle M, Arthur J, Anwanyu L et al. Keele University multicentre assisted delivery trial. In: 15th Annual Meeting of British Association of Perinatal Medicine; 1990; UK. 1990.
-
- Johanson RB, O'Brien PM, Rice C, Doyle M, Arthur J, Anwanyu L et al. The Keele University multicentre assisted delivery trial. In: 12th European Congress of Perinatal Medicine; 1990 Sept 11-14; Lyon, France. 1990:225.
-
- Johanson RB, Rice C, Doyle M, Arthur J, Anwanyu L, Ibrahim J, et al. Multicentre assisted delivery trial. British Journal of Obstetrics and Gynaecology 1992;99:268-9.
Kuit 1993 {published data only}
-
- Kuit JA, Eppinga HG, Wallenburg HC, Huikeshoven FJ. A randomized comparison of vacuum extraction delivery with a rigid and a pliable cup. Obstetrics & Gynecology 1993;82(2):280-4. - PubMed
-
- Kuit JA, Huikeshoven FJ, Eppinga HG, Wallenburg HC. A randomized comparative clinical study of soft cup and hard cup vacuum extraction. Nederlands Tijdschrift voor Obstetrie & Gynaecologie 1990;103:289-90.
-
- Kuit JA, Huikeshoven FJ, Eppinga HG, Wallenburg HC. Neonatal assessments after vacuum extraction - rigid versus flexible cup. In: 13th World Congress of Gynaecology and Obstetrics (FIGO); 1991 Sept 15-20; Singapore. 1991:33.
Lasbrey 1964 {published data only}
-
- Lasbrey AH, Orchard CD, Crichton D. A study of the relative merits and scope for vacuum extraction as opposed to forceps delivery. South African Journal of Obstetrics and Gynaecology 1964;2:1-3.
Lee 1996 {published data only}
-
- Lee HY, Subramaniam N, Nordin MM. Vacuum delivery at the maternity hospital Kuala Lumpur: a comparison of metal and silicone cups. Singapore Medical Journal 1996;37(1):55-60. - PubMed
Mola 2010 {published data only}
-
- Mola GD, Kuk JM. A randomised controlled trial of two instruments for vacuum-assisted delivery (vacca re-usable omnicup and the bird anterior and posterior cups) to compare failure rates, safety and use effectiveness. Australian and New Zealand Journal of Obstetrics and Gynaecology 2010;50(3):246-52. - PubMed
Pliego Perez 2000 {published data only}
-
- Pliego Perez AR, Moncada Navarro O, Neri Ruz ES, Velasco Pasillas M. Comparative assessment of efficacy and safety of assisted vaginal delivery with forceps and with vacuum extractor. Ginecologia y Obstetricia de Mexico 2000;68:453-9. - PubMed
Roshan 2005 {published data only}
-
- Petrikovsky B, Sichinava L, Roshanfekr D. Reinvented "soft" forceps - should they be applied [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S104.
-
- Roshan DF, Petrikovsky B, Sichinava L, Rudick BJ, Rebarber A, Bender SD. Soft forceps. International Journal of Gynecology & Obstetrics 2005;88(3):249-52. - PubMed
Shekhar 2013 {published data only}
Srisomboon 1998 {published data only}
-
- Srisomboon J, Piyamongkol W, Sahapong V, Mongkolchaipak S. Comparison of vacuum extraction delivery between the conventional metal cup and the new soft rubber cup. Journal of the Medical Association of Thailand 1998;8(7):480-6. - PubMed
Thiery 1987 {published data only}
-
- Thiery M, Van Den Broecke R, Kermans G, Parewijck W, Dhont M, Vanlancker M, et al. A randomized study of two cups for vacuum extraction. Journal of Perinatal Medicine 1987;15(2):129-36. - PubMed
-
- Thiery M, Van Den Broecke R, Kermans G, Parewijck W, Dhont M, Vanlancker M, et al. Can the vacuum extractor be improved? In: 11th European Congress of Perinatal Medicine; Rome, Italy. 1988.
-
- Thiery M, Van Den Broecke R, Kermans G, Vanhaesebrouck P, Derom R, Van Kets H, et al. Vacuum extraction: randomized comparison of two cup models. In: 10th European Congress of Perinatal Medicine; 1986 Aug 12-16; Leipzig, Germany. 1986:247.
-
- Van Den Broecke R, Thiery M, Kermans G. The usefulness of two types of suction cups: a randomized comparative trial. Tijdschrift voor Geneeskunde 1986;42:603-6.
Vacca 1983 {published data only}
-
- Carmody F, Grant AM, Mutch L, Vacca A, Chalmers I. Follow-up of babies delivered in a randomized controlled comparison of vacuum extraction and forceps delivery. Acta Obstetricia et Gynecologica Scandinavica 1986;65(7):763-6. - PubMed
-
- Garcia J, Anderson J, Vacca A, Elbourne DR, Grant AM, Chalmers I. Views of women and their medical and midwifery attendants about instrumental delivery using vacuum extraction and forceps. Journal of Psychosomatic Obstetrics and Gynaecology 1985;4:1-9.
-
- Vacca A, Grant AM, Wyatt G, Chalmers I. Portsmouth operative delivery trial: a comparison of vacuum extraction and forceps delivery. British Journal of Obstetrics and Gynaecology 1983;90(12):1107-12. - PubMed
-
- Vacca A, Grant AM. Portsmouth operative delivery trial. A randomised controlled trial to compare vacuum extraction with forceps delivery. European Journal of Obstetrics & Gynecology and Reproductive Biology 1983;15:305-9.
Warwick 1993 {published data only}
-
- Warwick AP, Doyle PM, Geetha T, Wilkinson P, Johanson RB, O'Brien PM. A random allocation comparison of silicone and santoprene soft vacuum extractor cups for assisted delivery. Journal of Obstetrics and Gynaecology 1993;13:337-9.
Weerasekera 2002 {published data only}
-
- Weerasekera DS, Premaratne S. A randomised prospective trial of the obstetric forceps versus vacuum extraction using defined criteria. Journal of Obstetrics and Gynaecology 2002;22(4):344-5. - PubMed
Williams 1991 {published data only}
-
- Williams MC, Knuppel RA, O'Brien WF, Weiss A, Kanarek KS. A randomized comparison of assisted vaginal delivery by obstetric forceps and polyethylene vacuum cup. Obstetrics & Gynecology 1991;78(5 Pt 1):789-94. - PubMed
-
- Williams MC, Knuppel RA, Weiss A, Kanarak N, O'Brien WF. A prospectively randomized comparison of forceps and vacuum assisted vaginal delivery. American Journal of Obstetrics and Gynecology 1991;164:323. - PubMed
References to studies excluded from this review
Carmona 1995 {published data only}
-
- Carmona F, Martinez-Roman S, Manau D, Cararach V, Iglesias X. Immediate maternal and neonatal effects of low-forceps delivery according to the new criteria of The American College of Obstetricians and Gynecologists compared with spontaneous vaginal delivery in term pregnancies. American Journal of Obstetrics and Gynecology 1995;173(1):55-9. - PubMed
Ehlers 1974 {published data only}
-
- Ehlers N, Krarup Jensen IB, Brogard Hansen K. Retinal haemorrhages in the newborn. Comparison of delivery by forceps and by vacuum extractor. Acta Ophthalmolologica 1974;52(1):73-82. - PubMed
Gabrawi 1997 {published data only}
-
- Gabrawi E, Johanson RB, Jones P. A random controlled trial of two different vacuum extractor pumps: new foot pump and electric pump. Journal of Obstetrics and Gynaecology 1997;17(4):325-7. - PubMed
George 1992 {published data only}
-
- George S. Trial of a newly-designed obstetric forceps. Personal communication 1992.
Katz 1982 {published data only}
-
- Katz Z, Lancet M, Dgani R, Ben-Hur H, Zalel Y. The beneficial effect of vacuum extraction on the fetus. Acta Obstetricia et Gynecologica Scandinavica 1982;61(4):337-40. - PubMed
Lim 1997 {published data only}
-
- Lim FH, Holm JP, Schuitemaker NE, Jansen FH, Hermans J. Stepwise compared with rapid application of vacuum in ventouse extraction procedures. British Journal of Obstetrics and Gynaecology 1997;104(1):33-6. - PubMed
Loghis 1992 {published data only}
-
- Loghis C, Pyrgiotis E, Panayotopoulos N, Batalias L, Salamalekis E, Zourlas PA. Comparison between metal cup and silicone rubber cup vacuum extractor. European Journal of Obstetrics & Gynecology and Reproductive Biology 1992;45(3):173-6. - PubMed
-
- Loghis C, Salamalekis E, Fotopoulis S, Panayotopoulos N, Zourlas PA. Comparison of assisted deliveries by forceps and silicone rubber cup vacuum extractor. In: 13th World Congress of Gynaecology and Obstetrics (FIGO); 1991 Sept 15-20; Singapore. 1991:64.
-
- Salamalekis E, Loghis C, Pyrgiotis E, Zourlas PA. Soft cup vacuum extractor vs forceps delivery. Journal of Obstetrics and Gynaecology 1995;15:245-6.
Maleckiene 1996 {published data only}
-
- Maleckiene L, Railaite DR. A randomized comparison of assisted vaginal delivery by vacuum extractor and obstetrics forceps. Prenatal and Neonatal Medicine 1996;1 Suppl 1:318.
Maltau 1984 {published data only}
-
- Maltau JM, Egge K, Moe N. Retinal hemorrhages in the preterm neonate. A prospective randomized study comparing the occurrence of hemorrhages after spontaneous vs forceps delivery. Acta Obstetricia et Gynecologica Scandinavica 1984;63(3):219-21. - PubMed
Mejido 2019 {published data only}
-
- Mejido J, ACTRN12618000355279. The correlation between the type of operative vaginal delivery (forceps or vacuum) and the rate of levator ani muscle avulsion: clinical trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12618000355279 (first received 16 October 2017).
-
- Mejido J, NCT03683264. Forceps vs vacuum. Rate of levator ani muscle avulsion: clinical trial. clinicaltrials.gov/ct2/show/NCT03683264 (first received 25 September 2018).
Mustafa 2002 {published data only}
-
- Mustafa R, Mustafa R. Perinatal and maternal outcome in ventouse versus forceps delivery. Journal of the College of Physicians & Surgeons Pakistan 2002;12(6):345-7.
Romero 2021 {published data only}
-
- Westgren M, NCT03071783. Intra-operative feed back on traction force during vacuum extraction: a randomized control study of mid and low metal cup deliveries. clinicaltrials.gov/ct2/show/record/NCT03071783 (first received 7 March 2017).
Schuitemaker 1992 {published data only}
-
- Schuitemaker NW. Trial to compare rapid vs conventional creation of negative pressure for vacuum extraction. Personal communication 1992.
Suwannachat 2011 {published data only}
-
- Suwannachat B, Laopaiboon M, Tonmat S, Siriwachirachai T, Teerapong S, Winiyakul N, et al. Rapid versus stepwise application of negative pressure in vacuum extraction-assisted vaginal delivery: a multicentre randomised controlled non-inferiority trial. British Journal of Obstetrics and Gynaecology 2011;118(10):1247-52. - PubMed
Williams 1993 {published data only}
Yancey 1991 {published data only}
-
- Yancey MK, Herpolsheimer A, Jordan GD, Benson WL, Brady K. Maternal and neonatal effects of outlet forceps delivery compared with spontaneous vaginal delivery in term pregnancies. Obstetrics & Gynecology 1991;78(4):646-50. - PubMed
References to ongoing studies
Schvartzman 2012 {published data only}
-
- Schvartzman JA, Carroli G, Di GC, Hofmeyr J, Kafrissen M, Merialdi M et al. The odon device. a new simple instrument for assisted vaginal delivery. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S475-6.
Additional references
Anode 2019
-
- Anode CG, Knight M. prophylactic antibiotics for the prevention of infection following operative vaginal delivery: the ANODE trial. American Journal of Obstetrics and Gynecology 2019;220(1):S685. - PubMed
Bailey 2017
-
- Bailey PE, Roosmalen J, Mola G, Evans C, De Bernia L, Dao B. Assisted vaginal delivery in low and middle income countries: an overview. BJOG: an international journal of obstetrics and gynaecology 2017;124(9):1335-44. [DOI: ] - PubMed
Bligard 2019
-
- Bligard K, Lipsey KL, Young OM. Simulation training for operative vaginal delivery among obstetrics and gynecology residents: a systematic review. Obstetrics and Gynecology 2019;134(Suppl 1):16S-21S. - PubMed
Chalmers 1989
-
- Chalmers I, Enkin M, Keirse MJ. Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1989.
EPOC 2018
-
- Cochrane Effective Practice and Organisation of Care (EPOC). Reporting the effects of an intervention in EPOC reviews. EPOC Resources for review authors, 2018. epoc.cochrane.org/resources/epoc-resourcesreview-authors (accessed 15 August 2020).
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors) Available from wwwtrainingcochraneorg/handbook. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). The Cochrane Collaboration, 2021. Available from www.training.cochrane.org/handbook. [978-1-119-53662-8]
Lapeer 2014
-
- Lapeer R, Audinis V, Gerikhanov Z, Dupuis O. A computer-based simulation of obstetric forceps placement. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2014;17(Pt 2):57-64. - PubMed
Liabsuetrakul 2020
Majoko 2012
NHS Maternity Statistics 2017
-
- NHS. Maternity Statistics, England. digital.nhs.uk/data-and-information/publications/statistical/nhs-materni... 2016-2017.
Nikpoor 2013
O'Brien 2019
O'Brien, 2017
O'Connell 2000
-
- O'Connell SW, Lindow M. Trends in obstetric care in the United Kingdom. Journal of Obstetrics and Gynaecology 2000;20(6):592-3. - PubMed
Patel 2004
RevMan 2020 [Computer program]
-
- Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Suwannachat 2012
WHO 2006
-
- World Health Organization. Neonatal and perinatal mortality: country, regional and global estimate. WHO (apps.who.int/iris/handle/10665/43444) (accessed 29th August 2020).
Wood 2017
-
- Wood SL, Tang S, Crawford S. Cesarean delivery in the second stage of labor and the risk of subsequent premature birth. American Journal of Obstetrics and Gynecology 2017;217(1):e1-63.e10. - PubMed
References to other published versions of this review
Johanson 1999
Johanson 2000
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

