Azithromycin for the prevention of rehospitalisation and death among Kenyan children being discharged from hospital: a double-blind, placebo-controlled, randomised controlled trial
- PMID: 34559992
- PMCID: PMC8638697
- DOI: 10.1016/S2214-109X(21)00347-8
Azithromycin for the prevention of rehospitalisation and death among Kenyan children being discharged from hospital: a double-blind, placebo-controlled, randomised controlled trial
Abstract
Background: Mass drug administration of azithromycin to children in sub-Saharan Africa has been shown to improve survival in high-mortality settings. The period after hospital discharge is a time of elevated risk unaddressed by current interventions and might provide an opportunity for targeting empirical azithromycin administration. We aimed to assess the efficacy of azithromycin administered at hospital discharge on risk of death and rehospitalisation in Kenyan children younger than 5 years.
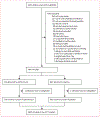
Methods: In this double-blind, placebo-controlled randomised trial, children were randomly assigned (1:1) to receive a 5-day course of azithromycin (oral suspension 10 mg/kg on day 1, followed by 5mg/kg per day on days 2-5) or identically appearing and tasting placebo at discharge from four hospitals in western Kenya. Children were eligible if they were aged 1-59 months at hospital discharge, weighed at least 2 kg, and had been admitted to hospital for any medical reason other than trauma, poisoning, or congenital anomaly. The primary outcome was death or rehospitalisation in the subsequent 6-month period in a modified intention-to-treat population, compared by randomisation group with Cox proportional hazards regression and Kaplan-Meier. Azithromycin resistance in Escherichia coli isolates from a random subset of children was compared by randomisation group with generalised estimating equations. This trial is registered with ClinicalTrials.gov, NCT02414399.
Findings: Between June 28, 2016, and Nov 4, 2019, 1400 children were enrolled in the trial at discharge from hospital, with 703 (50·2%) randomly assigned to azithromycin and 697 (49·8%) to placebo. Among the 1398 children included in the modified intention-to-treat analysis (702 in the azithromycin group and 696 in the placebo group), the incidence of death or rehospitalisation was 20·4 per 100 child-years in the azithromycin group and 22·5 per 100 child-years in the placebo group (adjusted hazard ratio 0·91, 95·5% CI 0·64-1·29, p=0·58). Azithromycin resistance was common in commensal E coli isolates from enrolled children before randomisation (37·7% of 406 isolates) despite only 3·7% of children having received a macrolide antibiotic during the hospitalisation. Azithromycin resistance was slightly higher at 3 months after randomisation in the azithromycin group (26·9%) than in the placebo group (19·1%; adjusted prevalence ratio 1·41, 95% CI 0·95-2·09, p=0·088), with no difference observed at 6 months (1·17, 0·78-1·76, p=0·44).
Interpretation: We did not observe a significant benefit of a 5-day course of azithromycin delivered to children younger than 5 years at hospital discharge despite the overall high risk of mortality and rehospitalisation. These findings highlight the need for more research into mechanisms and interventions for prevention of morbidity and mortality in the post-discharge period.
Funding: Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests BAR reports participation on Data Safety and Monitoring Boards for HIV PrEP clinical trials funded by Gilead and COVID-19 treatment trials funded by the US National Institute of Allergy and Infectious Diseases, outside the submitted work. All other authors declare no competing interests.
Figures


Comment in
-
MDA and trial designs to evaluate the impact of azithromycin on child mortality.Lancet Glob Health. 2022 Feb;10(2):e183. doi: 10.1016/S2214-109X(21)00557-X. Lancet Glob Health. 2022. PMID: 35063109 No abstract available.
References
-
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2016 results. Seattle, WA: Institute for Health Metrics and Evaluation, 2017.
-
- WHO. WHO guideline on mass drug administration of azithromycin to children under five years of age to promote child survival. Geneva: World Health Organization, 2020. - PubMed
-
- Coles CL, Mabula K, Seidman JC, et al. Mass distribution of azithromycin for trachoma control is associated with increased risk of azithromycin-resistant Streptococcus pneumoniae carriage in young children 6 months after treatment. Clin Infect Dis 2013; 56: 1519–26. - PubMed
-
- Haug S, Lakew T, Habtemariam G, et al. The decline of pneumococcal resistance after cessation of mass antibiotic distributions for trachoma. Clin Infect Dis 2010; 51: 571–74. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

