Access to cancer medicines deemed essential by oncologists in 82 countries: an international, cross-sectional survey
- PMID: 34560006
- PMCID: PMC8476341
- DOI: 10.1016/S1470-2045(21)00463-0
Access to cancer medicines deemed essential by oncologists in 82 countries: an international, cross-sectional survey
Abstract
Background: The WHO Essential Medicines List (EML) identifies priority medicines that are most important to public health. Over time, the EML has included an increasing number of cancer medicines. We aimed to investigate whether the cancer medicines in the EML are aligned with the priority medicines of frontline oncologists worldwide, and the extent to which these medicines are accessible in routine clinical practice.
Methods: This international, cross-sectional survey was developed by investigators from a range of clinical practice settings across low-income to high-income countries, including members of the WHO Essential Medicines Cancer Working Group. A 28-question electronic survey was developed and disseminated to a global network of oncologists in 89 countries and regions by use of a hierarchical snowball method; each primary contact distributed the survey through their national and regional oncology associations or personal networks. The survey was open from Oct 15 to Dec 7, 2020. Fully qualified physicians who prescribe systemic anticancer therapy to adults were eligible to participate in the survey. The primary question asked respondents to select the ten cancer medicines that would provide the greatest public health benefit to their country; subsequent questions explored availability and cost of cancer medicines. Descriptive statistics were used to compare access to medicines between low-income and lower-middle-income countries, upper-middle-income countries, and high-income countries.
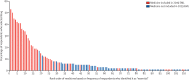
Findings: 87 country-level contacts and two regional networks were invited to participate in the survey; 46 (52%) accepted the invitation and distributed the survey. 1697 respondents opened the survey link; 423 were excluded as they did not answer the primary study question and 326 were excluded because of ineligibility. 948 eligible oncologists from 82 countries completed the survey (165 [17%] in low-income and lower-middle-income countries, 165 [17%] in upper-middle-income countries, and 618 [65%] in high-income countries). The most commonly selected medicines were doxorubicin (by 499 [53%] of 948 respondents), cisplatin (by 470 [50%]), paclitaxel (by 423 [45%]), pembrolizumab (by 414 [44%]), trastuzumab (by 402 [42%]), carboplatin (by 390 [41%]), and 5-fluorouracil (by 386 [41%]). Of the 20 most frequently selected high-priority cancer medicines, 19 (95%) are currently on the WHO EML; 12 (60%) were cytotoxic agents and 13 (65%) were granted US Food and Drug Administration regulatory approval before 2000. The proportion of respondents indicating universal availability of each top 20 medication was 9-54% in low-income and lower-middle-income countries, 13-90% in upper-middle-income countries, and 68-94% in high-income countries. The risk of catastrophic expenditure (spending >40% of total consumption net of spending on food) was more common in low-income and lower-middle-income countries, with 13-68% of respondents indicating a substantial risk of catastrophic expenditures for each of the top 20 medications in lower-middle-income countries versus 2-41% of respondents in upper-middle-income countries and 0-9% in high-income countries.
Interpretation: These data demonstrate major barriers in access to core cancer medicines worldwide. These findings challenge the feasibility of adding additional expensive cancer medicines to the EML. There is an urgent need for global and country-level policy action to ensure patients with cancer globally have access to high priority medicines.
Funding: None.
© 2021 World Health Organization; licensee Elsevier. This is an Open Access article published under the CC BY 3.0 IGO license which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In any use of this article, there should be no suggestion that WHO endorses any specific organisation, products or services. The use of the WHO logo is not permitted. This notice should be preserved along with the article's original URL.
Conflict of interest statement
Declaration of interests FR, LM, and AI are employees of WHO. MS, DL, LM, EGEDV, DT, FR, RS, BG, and CMB are members of the WHO Essential Medicines List Cancer Medicine Working Group. EGEDV reports institutional financial support for research from Amgen, Bayer, Crescendo Biologics, CytomX Therapeutics, G1 Therapeutics, Genentech, Regeneron, Roche, Servier, and Synthon, and institutional financial support for participation on data safety monitoring boards and advisory boards of Daiichi Sankyo, NSABP, and Crescendo Biologics. FR reports honoraria for lectures from Dr Reddy's and payments for participation on advisory boards from Boehringer-Ingelheim and MSD. BG reports consulting fees from Vivio Health. All other authors declare no competing interests.
Figures
Comment in
-
Access to and affordability of cancer medicines: time to focus on the last mile.Lancet Oncol. 2021 Oct;22(10):1342-1343. doi: 10.1016/S1470-2045(21)00518-0. Epub 2021 Sep 21. Lancet Oncol. 2021. PMID: 34560007 No abstract available.
-
Prioritising access to cancer drugs.Lancet Oncol. 2022 Jan;23(1):e1. doi: 10.1016/S1470-2045(21)00641-0. Lancet Oncol. 2022. PMID: 34973219 No abstract available.
-
Prioritising access to cancer drugs.Lancet Oncol. 2022 Jan;23(1):e2. doi: 10.1016/S1470-2045(21)00637-9. Lancet Oncol. 2022. PMID: 34973227 No abstract available.
References
-
- WHO Expert Committee on the Selection of Essential Drugs & World Health Organization The selection of essential drugs: report of a WHO expert committee [meeting held in Geneva from 17 to 21 October 1977] 1977. https://apps.who.int/iris/handle/10665/41272
-
- WHO The selection and use of essential medicines: report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2019 (including the 21st WHO Model List of Essential Medicines and the 7th WHO Model List of Essential Medicines for Children) 2019. https://apps.who.int/iris/bitstream/handle/10665/330668/9789241210300-en...
-
- WHO Selection of essential medicines at country level: using the WHO Model List of Essential Medicines to update a national essential medicines list. 2019. https://apps.who.int/iris/bitstream/handle/10665/330668/9789241210300-en...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

