CD19-targeted chimeric antigen receptor T-cell therapy for CNS relapsed or refractory acute lymphocytic leukaemia: a post-hoc analysis of pooled data from five clinical trials
- PMID: 34560014
- PMCID: PMC9026766
- DOI: 10.1016/S2352-3026(21)00238-6
CD19-targeted chimeric antigen receptor T-cell therapy for CNS relapsed or refractory acute lymphocytic leukaemia: a post-hoc analysis of pooled data from five clinical trials
Erratum in
-
Correction to Lancet Haematol 2021; 8: e711-22.Lancet Haematol. 2021 Nov;8(11):e789. doi: 10.1016/S2352-3026(21)00309-4. Lancet Haematol. 2021. PMID: 34715045 No abstract available.
Abstract
Background: CNS relapse of acute lymphocytic leukaemia is difficult to treat. Durable remissions of relapsed or refractory B-cell acute lymphocytic leukaemia have been observed following treatment with CD19-directed chimeric antigen receptor (CAR) T cells; however, most trials have excluded patients with active CNS disease. We aimed to assess the safety and activity of CAR T-cell therapy in patients with a history of CNS relapsed or refractory B-cell acute lymphocytic leukaemia.
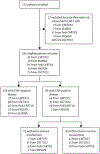
Methods: In this post-hoc analysis, we included 195 patients (aged 1-29 years; 110 [56%] male and 85 [44%] female) with relapsed or refractory CD19-positive acute lymphocytic leukaemia or lymphocytic lymphoma from five clinical trials (Pedi CART19, 13BT022, ENSIGN, ELIANA, and 16CT022) done at the Children's Hospital of Philadelphia (Philadelphia, PA, USA), in which participants received CD19-directed CAR T-cell therapy between April 17, 2012, and April 16, 2019. The trials required control of CNS disease at enrolment and infusion and excluded treatment in the setting of acute neurological toxic effects (>grade 1 in severity) or parenchymal lesions deemed to increase the risk of neurotoxicity. 154 patients from Pedi CART19, ELIANA, ENSIGN, and 16CT022 received tisagenlecleucel and 41 patients from the 13BT022 trial received the humanised CD19-directed CAR, huCART19. We categorised patients into two strata on the basis of CNS status at relapse or within the 12 months preceding CAR T-cell infusion-either CNS-positive or CNS-negative disease. Patients with CNS-positive disease were further divided on the basis of morphological bone marrow involvement-either combined bone marrow and CNS involvement, or isolated CNS involvement. Endpoints were the proportion of patients with complete response at 28 days after infusion, Kaplan-Meier analysis of relapse-free survival and overall survival, and the incidence of cytokine release syndrome and neurotoxicity.
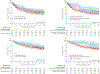
Findings: Of all 195 patients, 66 (34%) were categorised as having CNS-positive disease and 129 (66%) as having CNS-negative disease, and 43 (22%) were categorised as having isolated CNS involvement. The median length of follow-up was 39 months (IQR 25-49) in the CNS-positive stratum and 36 months (18-49) in the CNS-negative stratum. The proportion of patients in the CNS-positive stratum with a complete response at 28 days after infusion was similar to that in the CNS-negative stratum (64 [97%] of 66 vs 121 [94%] of 129; p=0·74), with no significant difference in relapse-free survival (60% [95% CI 49-74] vs 60% [51-71]; p=0·50) or overall survival (83% [75-93] vs 71% [64-79]; p=0·39) at 2 years between the two groups. Overall survival at 2 years was significantly higher in patients with isolated CNS involvement compared with those with bone marrow involvement (91% [82-100] vs 71% [64-78]; p=0·046). The incidence and severity of neurotoxicity (any grade, 53 [41%] vs 38 [58%]; grade 1, 24 [19%] vs 20 [30%]; grade 2, 14 [11%] vs 10 [15%]; grade 3, 12 [9%] vs 6 [9%], and grade 4, 3 [2%] vs 2 [3%]; p=0·20) and cytokine release syndrome (any grade, 110 [85%] vs 53 [80%]; grade 1, 12 [9%] vs 2 [3%]; grade 2, 61 [47%] vs 38 [58%]; grade 3, 18 [14%] vs 7 [11%] and grade 4, 19 [15%] vs 6 [9%]; p=0·26) did not differ between the CNS-negative and the CNS-positive disease strata.
Interpretation: Tisagenlecleucel and huCART19 are active at clearing CNS disease and maintaining durable remissions in children and young adults with CNS relapsed or refractory B-cell acute lymphocytic leukaemia or lymphocytic lymphoma, without increasing the risk of severe neurotoxicity; although care should be taken in the timing of therapy and disease control to mitigate this risk. These preliminary findings support the use of these CAR T-cell therapies for patients with CNS relapsed or refractory B-cell acute lymphocytic leukaemia.
Funding: Children's Hospital of Philadelphia Frontier Program.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests CC has served as a consultant for Novartis Pharmaceuticals. CHJ is an inventor of intellectual property, licensed by the University of Pennsylvania to Novartis; has received patent royalties; is a scientific co-founder of Tmunity Therapeutics and DeCART Therapeutics, for which he has founder's stock but no income; and is an advisor for AC Immune, Bluesphere Bio, Cellares, Celldex, Cabaletta, Carisma, Kiadis, Viracta, and Ziopharm. SAG has received research or clinical trial support, or both, from Novartis, Servier, and Kite, and has participated in consulting, in study steering committees, or in scientific or clinical advisory boards for Novartis, Cellectis, Adaptimmune, Eureka, TCR2, Juno, GlaxoSmithKline, Vertex, Humanigen, CBMG, Janssen/JnJ, Jazz Pharmaceuticals, Allogene, Cabaletta, and Roche. SRR has received research funding from and has served as a consultant for Pfizer, and a family member owns stock in Optinose. SLM has served as a consultant for Novartis Pharmaceuticals, Kite Pharma, and Wugen, and receives clinical trial funding from Novartis Pharmaceuticals. All other authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

