Probing the molecular basis for signal transduction through the Zinc-Activated Channel (ZAC)
- PMID: 34560053
- PMCID: PMC11359809
- DOI: 10.1016/j.bcp.2021.114781
Probing the molecular basis for signal transduction through the Zinc-Activated Channel (ZAC)
Abstract
The molecular basis for the signal transduction through the classical Cys-loop receptors (CLRs) has been delineated in great detail. The Zinc-Activated Channel (ZAC) constitutes a so far poorly elucidated fifth branch of the CLR superfamily, and in this study we explore the molecular mechanisms underlying ZAC signaling in Xenopus oocytes by two-electrode voltage clamp electrophysiology. In studies of chimeric receptors fusing either the extracellular domain (ECD) or the transmembrane/intracellular domain (TMD-ICD) of ZAC with the complementary domains of 5-HT3A serotonin or α1 glycine receptors, serotonin and Zn2+/H+ evoked robust concentration-dependent currents in 5-HT3A/ZAC- and ZAC/α1-Gly-expressing oocytes, respectively, suggesting that Zn2+ and protons activate ZAC predominantly through its ECD. The molecular basis for Zn2+-mediated ZAC signaling was probed further by introduction of mutations of His, Cys, Glu and Asp residues in this domain, but as none of the mutants tested displayed substantially impaired Zn2+ functionality compared to wild-type ZAC, the location of the putative Zn2+ binding site(s) in the ECD was not identified. Finally, the functional importance of Leu246 (Leu9') in the transmembrane M2 α-helix of ZAC was investigated by Ala, Val, Ile and Thr substitutions. In concordance with findings for this highly conserved residue in classical CLRs, the ZACL9'X mutants exhibited left-shifted agonist concentration-response relationships, markedly higher degrees of spontaneous activity and slower desensitization kinetics compared to wild-type ZAC. In conclusion, while ZAC is an atypical CLR in terms of its (identified) agonists and channel characteristics, its signal transduction seems to undergo similar conformational transitions as those in the classical CLR.
Keywords: Agonist binding; Chimeric subunits; Cys-loop receptor (CLR); Leu9′ residue; Pentameric ligand-gated ion channel (pLGIC); Zinc-Activated Channel (ZAC).
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
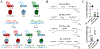





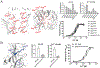



References
-
- Taly A, Corringer P-J, Guedin D, Lestage P, Changeux J-P, Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system, Nat Rev Drug Discov 8 (9) (2009) 733–750. - PubMed
-
- Bertrand D, Lee C-H, Flood D, Marger F, Donnelly-Roberts D, Esbenshade TA, Therapeutic Potential of α7 Nicotinic Acetylcholine Receptors, Pharmacol Rev 67 (4) (2015) 1025–1073. - PubMed
-
- Grupe M, Grunnet M, Bastlund JF, Jensen AA, Targeting α4β2 nicotinic acetylcholine receptors in central nervous system disorders: perspectives on positive allosteric modulation as a therapeutic approach, Basic Clin Pharmacol Toxicol 116 (3) (2015) 187–200. - PubMed
-
- Walstab J, Rappold G, Niesler B, 5-HT3 receptors: role in disease and target of drugs, Pharmacol Ther 128 (1) (2010) 146–169. - PubMed
-
- Fakhfouri G, Rahimian R, Dyhrfjeld-Johnsen J, Zirak MR, Beaulieu J-M, Witkin JM, 5-HT3 receptor antagonists in neurologic and neuropsychiatric disorders: The iceberg still lies beneath the surface, Pharmacol Rev 71 (3) (2019) 383–412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

