Collagen XIV Is an Intrinsic Regulator of Corneal Stromal Structure and Function
- PMID: 34560063
- PMCID: PMC8696667
- DOI: 10.1016/j.ajpath.2021.08.016
Collagen XIV Is an Intrinsic Regulator of Corneal Stromal Structure and Function
Abstract
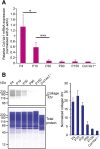
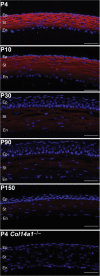
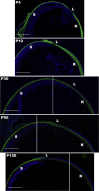
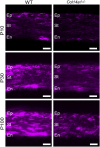
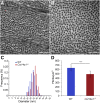
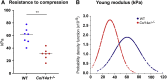
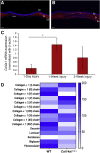
Collagen XIV is poorly characterized in the body, and the current knowledge of its function in the cornea is limited. The aim of the current study was to elucidate the role(s) of collagen XIV in regulating corneal stromal structure and function. Analysis of collagen XIV expression, temporal and spatial, was performed at different postnatal days (Ps) in wild-type C57BL/6 mouse corneal stromas and after injury. Conventional collagen XIV null mice were used to inquire the roles that collagen XIV plays in fibrillogenesis, fibril packing, and tissue mechanics. Fibril assembly and packing as well as stromal organization were evaluated using transmission electron microscopy and second harmonic generation microscopy. Atomic force microscopy was used to assess stromal stiffness. Col14a1 mRNA expression was present at P4 to P10 and decreased at P30. No immunoreactivity was noted at P150. Abnormal collagen fibril assembly with a shift toward larger-diameter fibrils and increased interfibrillar spacing in the absence of collagen XIV was found. Second harmonic generation microscopy showed impaired fibrillogenesis in the collagen XIV null stroma. Mechanical testing suggested that collagen XIV confers stiffness to stromal tissue. Expression of collagen XIV is up-regulated following injury. This study indicates that collagen XIV plays a regulatory role in corneal development and in the function of the adult cornea. The expression of collagen XIV is recapitulated during wound healing.
Copyright © 2021 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Abnormal corneal endothelial maturation in collagen XII and XIV null mice.Invest Ophthalmol Vis Sci. 2013 May 7;54(5):3297-308. doi: 10.1167/iovs.12-11456. Invest Ophthalmol Vis Sci. 2013. PMID: 23599329 Free PMC article.
-
Type XIV Collagen Regulates Fibrillogenesis: PREMATURE COLLAGEN FIBRIL GROWTH AND TISSUE DYSFUNCTION IN NULL MICE.J Biol Chem. 2009 Mar 27;284(13):8427-38. doi: 10.1074/jbc.M805582200. Epub 2009 Jan 9. J Biol Chem. 2009. PMID: 19136672 Free PMC article.
-
The roles of types XII and XIV collagen in fibrillogenesis and matrix assembly in the developing cornea.J Cell Biochem. 2002;87(2):208-20. doi: 10.1002/jcb.10290. J Cell Biochem. 2002. PMID: 12244573
-
Regulation of corneal stroma extracellular matrix assembly.Exp Eye Res. 2015 Apr;133:69-80. doi: 10.1016/j.exer.2014.08.001. Exp Eye Res. 2015. PMID: 25819456 Free PMC article. Review.
-
Composition, structure and function of the corneal stroma.Exp Eye Res. 2020 Sep;198:108137. doi: 10.1016/j.exer.2020.108137. Epub 2020 Jul 11. Exp Eye Res. 2020. PMID: 32663498 Free PMC article. Review.
Cited by
-
Collagen XII regulates stromal wound closure.Exp Eye Res. 2023 May;230:109456. doi: 10.1016/j.exer.2023.109456. Epub 2023 Mar 24. Exp Eye Res. 2023. PMID: 36967080 Free PMC article.
-
Genipin increases extracellular matrix synthesis preventing corneal perforation.Ocul Surf. 2023 Apr;28:115-123. doi: 10.1016/j.jtos.2023.02.003. Epub 2023 Mar 3. Ocul Surf. 2023. PMID: 36871831 Free PMC article.
-
Collagen type XIV is proportionally lower in the lung tissue of patients with IPF.Sci Rep. 2023 Nov 8;13(1):19393. doi: 10.1038/s41598-023-46733-5. Sci Rep. 2023. PMID: 37938243 Free PMC article.
-
Corneal stromal repair and regeneration.Prog Retin Eye Res. 2022 Nov;91:101090. doi: 10.1016/j.preteyeres.2022.101090. Epub 2022 May 29. Prog Retin Eye Res. 2022. PMID: 35649962 Free PMC article. Review.
-
Downregulation of collagen XI during late postnatal corneal development is followed by upregulation after injury.J Cell Sci. 2022 Jan 1;135(1):jcs258694. doi: 10.1242/jcs.258694. Epub 2022 Jan 12. J Cell Sci. 2022. PMID: 34854919 Free PMC article.
References
-
- Mienaltowski M.J., Birk D.E. Structure, physiology, and biochemistry of collagens. Adv Exp Med Biol. 2014;802:5–29. - PubMed
-
- Gordon M.K., Castagnola P., Dublet B., Linsenmayer T.F., Van der Rest M., Mayne R., Olsen B.R. Cloning of a cDNA for a new member of the class of fibril-associated collagens with interrupted triple helices. Eur J Biochem. 1991;201:333–338. - PubMed
-
- Shaw L.M., Olsen B.R. FACIT collagens: diverse molecular bridges in extracellular matrices. Trends Biochem Sci. 1991;16:191–194. - PubMed
-
- Agarwal P., Zwolanek D., Keene D.R., Schulz J.N., Blumbach K., Heinegard D., Zaucke F., Paulsson M., Krieg T., Koch M., Eckes B. Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure. J Biol Chem. 2012;287:22549–22559. - PMC - PubMed
-
- Walchli C., Trueb J., Kessler B., Winterhalter K.H., Trueb B. Complete primary structure of chicken collagen XIV. Eur J Biochem. 1993;212:483–490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

