Comparing the diagnostic accuracy of rapid antigen detection tests to real time polymerase chain reaction in the diagnosis of SARS-CoV-2 infection: A systematic review and meta-analysis
- PMID: 34560340
- PMCID: PMC8444381
- DOI: 10.1016/j.jcv.2021.104985
Comparing the diagnostic accuracy of rapid antigen detection tests to real time polymerase chain reaction in the diagnosis of SARS-CoV-2 infection: A systematic review and meta-analysis
Abstract
Background: Timely and accurate diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is crucial to reduce the risk of viral transmission. We investigated the diagnostic accuracy of rapid antigen detection tests (RADTs) in the diagnosis of SARS-CoV-2 infection.
Methods: A systematic literature search was performed using Pubmed, Embase, and the Cochrane Central Register. The sensitivity, specificity, diagnostic odds ratio (DOR), and a hierarchical summary receiver-operating characteristic curve (HSROC) of RADTs were pooled using meta-analysis. We used commercial and laboratory-developed reverse transcriptase-polymerase chain reaction (RT-PCR) as reference standards.
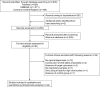
Results: We identified 24 studies comprising 14,188 patients. The overall pooled sensitivity, specificity, and DOR of RADTs for diagnosis of SARS-CoV-2 were 0.68 (95%CI, 0.59 - 0.76), 0.99 (95%CI, 0.99 - 1.00), and 426.70 (95% CI, 168.37 - 1081.65), respectively. RADTs and RT-PCR had moderate agreement with an estimated pooled Cohen's kappa statistic of 0.75 (95%CI, 0.74-0.77), and area under the HSROC of 0.98 (95%CI, 0.96 - 0.99). The pooled sensitivity of RADTs was significantly increased in subjects with viral load of Ct-value ≤25 or in those within 5 days after symptom onset than it was in subjects with lower viral loads or longer symptom duration.
Conclusions: The overall sensitivity of RADTs was inferior to that of the RT-PCR assay. The RADTs were more sensitive for samples of Ct-value ≤ 25 and might be suitable for subjects in the community within 5 days of symptom onset.
Keywords: Antigens; COVID-19 testing; Diagnosis; Point-of-care testing; SARS-CoV-2.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Figures



References
-
- Rong X.M., Yang L., Chu H.D., Fan M. Effect of delay in diagnosis on transmission of COVID-19. Mathematical biosciences and engineering. Math Biosci. Eng. 2020;17:2725–2740. - PubMed
-
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro surveillance. 2020;25 - PMC - PubMed
-
- World health organization, Coronavirus Disease (COVID-19) Pandemic — Emergency Use Listing Procedure (EUL) Open for IVDs, Available from: https://extranet.who.int/pqweb/vitro-diagnostics/coronavirus-disease-cov..., 2021 (accessed 19 Aug 2021).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

