MEST promotes lung cancer invasion and metastasis by interacting with VCP to activate NF-κB signaling
- PMID: 34560900
- PMCID: PMC8464132
- DOI: 10.1186/s13046-021-02107-1
MEST promotes lung cancer invasion and metastasis by interacting with VCP to activate NF-κB signaling
Abstract
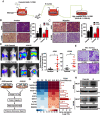
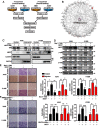
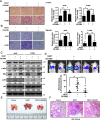
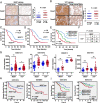
Background: Cell invasion is a hallmark of metastatic cancer, leading to unfavorable clinical outcomes. In this study, we established two highly invasive lung cancer cell models (A549-i8 and H1299-i8) and identified mesoderm-specific transcript (MEST) as a novel invasive regulator of lung cancer. We aim to characterize its biological function and clinical significance in lung cancer metastasis.
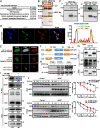
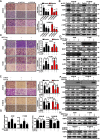
Methods: Transwell invasion assay was performed to establish high-invasive lung cancer cell model. Immunohistochemistry (IHC) was used to detect MEST expression in tumor tissues. Mass spectrometry and bioinformatic analyses were used to identify MEST-regulated proteins and binding partners. Co-immunoprecipitation assay was performed to detect the interaction of MEST and VCP. The biological functions of MEST were investigated in vitro and in vivo. Immunofluorescence staining was conducted to explore the colocalization of MEST and VCP.
Results: MEST overexpression promoted metastasis of lung cancer cells in vivo and in vitro by activating NF-κB signaling. MEST increased the interaction between VCP and IκBα, which accelerated IκBα degradation and NF-κB activation. Such acceleration was abrogated by VCP silencing, indicating that MEST is an upstream activator of the VCP/IκBα/NF-κB signaling pathway. Furthermore, high expressions of MEST and VCP were associated with poor survival of lung cancer patients.
Conclusion: Collectively, these results demonstrate that MEST plays an important role in driving invasion and metastasis of lung cancer by interacting with VCP to coordinate the IκBα/NF-κB pathway. Targeting the MEST/VCP/IκBα/NF-κB signaling pathway may be a promising strategy to treat lung cancer.
Keywords: Lung cancer; MEST; Metastasis; NF-κB; VCP.
© 2021. The Author(s).
Conflict of interest statement
The corresponding author and all the co-authors have agreed to the publication of the manuscript to Journal of Experimental and Clinical Cancer Research as a research article and declare that they have no conflict of interest as to the results presented.
The authors declare that they have no competing interests.
Figures


Similar articles
-
UBE2S activates NF-κB signaling by binding with IκBα and promotes metastasis of lung adenocarcinoma cells.Cell Oncol (Dordr). 2021 Dec;44(6):1325-1338. doi: 10.1007/s13402-021-00639-4. Epub 2021 Sep 28. Cell Oncol (Dordr). 2021. PMID: 34582005
-
lncRNA-PLACT1 sustains activation of NF-κB pathway through a positive feedback loop with IκBα/E2F1 axis in pancreatic cancer.Mol Cancer. 2020 Feb 21;19(1):35. doi: 10.1186/s12943-020-01153-1. Mol Cancer. 2020. PMID: 32085715 Free PMC article.
-
cIAP2 via NF-κB signalling affects cell proliferation and invasion in hepatocellular carcinoma.Life Sci. 2021 Feb 1;266:118867. doi: 10.1016/j.lfs.2020.118867. Epub 2020 Dec 10. Life Sci. 2021. PMID: 33310033
-
Valosin Containing Protein (VCP): A Multistep Regulator of Autophagy.Int J Mol Sci. 2022 Feb 9;23(4):1939. doi: 10.3390/ijms23041939. Int J Mol Sci. 2022. PMID: 35216053 Free PMC article. Review.
-
NF-kappaB fans the flames of lung carcinogenesis.Cancer Prev Res (Phila). 2010 Apr;3(4):403-5. doi: 10.1158/1940-6207.CAPR-10-0042. Epub 2010 Mar 30. Cancer Prev Res (Phila). 2010. PMID: 20354166 Free PMC article. Review.
Cited by
-
Semaphorin4A promotes lung cancer by activation of NF-κB pathway mediated by PlexinB1.PeerJ. 2023 Oct 24;11:e16292. doi: 10.7717/peerj.16292. eCollection 2023. PeerJ. 2023. PMID: 37901456 Free PMC article.
-
Characterization of 3D-Bioprinted In Vitro Lung Cancer Models Using RNA-Sequencing Techniques.Bioengineering (Basel). 2023 Jun 1;10(6):667. doi: 10.3390/bioengineering10060667. Bioengineering (Basel). 2023. PMID: 37370598 Free PMC article.
-
Multiple signaling pathways in the frontiers of lung cancer progression.Front Immunol. 2025 Jun 10;16:1593793. doi: 10.3389/fimmu.2025.1593793. eCollection 2025. Front Immunol. 2025. PMID: 40557151 Free PMC article. Review.
-
A hypoxia-derived gene signature to suggest cisplatin-based therapeutic responses in patients with cervical cancer.Comput Struct Biotechnol J. 2024 Jun 8;23:2565-2579. doi: 10.1016/j.csbj.2024.06.007. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 38983650 Free PMC article.
-
Inhibition of VCP modulates NF-κB signaling pathway to suppress multiple myeloma cell proliferation and osteoclast differentiation.Aging (Albany NY). 2023 Aug 21;15(16):8220-8236. doi: 10.18632/aging.204965. Epub 2023 Aug 21. Aging (Albany NY). 2023. PMID: 37606987 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

