Disparities in the participation and adherence of older adults in lifestyle-based multidomain dementia prevention and the motivational role of perceived disease risk and intervention benefits: an observational ancillary study to a randomised controlled trial
- PMID: 34560903
- PMCID: PMC8464095
- DOI: 10.1186/s13195-021-00904-6
Disparities in the participation and adherence of older adults in lifestyle-based multidomain dementia prevention and the motivational role of perceived disease risk and intervention benefits: an observational ancillary study to a randomised controlled trial
Abstract
Background: Preventive interventions for dementia are urgently needed and must be tested in randomised controlled trials (RCTs). Selection (volunteer) bias may limit efficacy, particularly in trials testing multidomain interventions and may also be indicative of disparities in intervention uptake in real-world settings. We identified factors associated with participation and adherence in a 3-year RCT of multidomain lifestyle intervention and/or omega-3 supplementation for prevention of cognitive decline and explored reasons for (non-) participation.
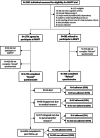
Methods: Ancillary study during recruitment and follow-up of the 3-year Multidomain Alzheimer Preventive Trial (MAPT) conducted in in 13 memory centres in France and Monaco, involving 1630 community-dwelling dementia-free individuals aged ≥ 70 who were pre-screened for MAPT (1270 participated in MAPT; 360 declined to participate).
Results: Response rates were 76% amongst MAPT participants and 53% amongst non-participants. Older individuals (odds ratio 0.94 [95% confidence interval 0.91-0.98] and those with higher anxiety (0.61 [0.47-0.79]) were less likely to participate in the trial. Those with higher income (4.42 [2.12-9.19]) and family history (1.60 [1.10-2.32]) or greater fear (1.73 [1.30-2.29]) of dementia were more likely to participate, as were those recruited via an intermediary (e.g. pension funds, local Alzheimer's associations, University of the 3rd Age, sports clubs) (2.15 [1.45-3.20]). MAPT participants living in larger towns (0.71 [0.55-0.92]) and with higher depressive symptoms (0.94 [0.90-0.99]) were less likely to adhere to the interventions. Greater perceived social support (1.21 [1.03-1.43]) and cognitive function (1.37 [1.13-1.67]) predicted better adherence. Descriptively, the most frequent reasons for accepting and refusing to participate were, respectively, altruism and logistical constraints, but underlying motivations mainly related to (lack of) perceived benefits.
Conclusions: Disparities in uptake of health interventions persist in older age. Those most at risk of dementia may not participate in or adhere to preventive interventions. Barriers to implementing lifestyle changes for dementia prevention include lack of knowledge about potential benefits, lack of support networks, and (perceived) financial costs.
Trial registration: NCT00672685 (ClinicalTrials.gov).
Keywords: Adherence; Disparities; Engagement; Intervention; Lifestyle; Multidomain; Participation; Population bias; Prevention.
© 2021. The Author(s).
Conflict of interest statement
SA reports grants from EU programs, Fondation de l’Avenir, and the AMPA and France Alzheimer Associations; personal fees from Nestlé, Nestec SA, Sanofi, and MSD; and non-financial support from Biogen, Pfizer, and Icon outside the submitted work. The other authors have no competing interests.
Figures

References
-
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. - DOI - PMC - PubMed
-
- Andrieu S, Guyonnet S, Coley N, Cantet C, Bonnefoy M, Bordes S, Bories L, Cufi MN, Dantoine T, Dartigues JF, Desclaux F, Gabelle A, Gasnier Y, Pesce A, Sudres K, Touchon J, Robert P, Rouaud O, Legrand P, Payoux P, Caubere JP, Weiner M, Carrié I, Ousset PJ, Vellas B, Vellas B, Guyonnet S, Carrié I, Brigitte L, Faisant C, Lala F, Delrieu J, Villars H, Combrouze E, Badufle C, Zueras A, Andrieu S, Cantet C, Morin C, van Kan GA, Dupuy C, Rolland Y, Caillaud C, Ousset PJ, Fougère B, Willis S, Belleville S, Gilbert B, Fontaine F, Dartigues JF, Marcet I, Delva F, Foubert A, Cerda S, Noëlle-Cuffi M, Costes C, Rouaud O, Manckoundia P, Quipourt V, Marilier S, Franon E, Bories L, Pader ML, Basset MF, Lapoujade B, Faure V, Li M, Tong Y, Malick-Loiseau C, Cazaban-Campistron E, Desclaux F, Blatge C, Dantoine T, Laubarie-Mouret C, Saulnier I, Clément JP, Picat MA, Bernard-Bourzeix L, Willebois S, Désormais I, Cardinaud N, Bonnefoy M, Livet P, Rebaudet P, Gédéon C, Burdet C, Terracol F, Pesce A, Roth S, Chaillou S, Louchart S, Sudres K, Lebrun N, Barro-Belaygues N, Touchon J, Bennys K, Gabelle A, Romano A, Touati L, Marelli C, Pays C, Robert P, le Duff F, Gervais C, Gonfrier S, Gasnier Y, Bordes S, Begorre D, Carpuat C, Khales K, Lefebvre JF, el Idrissi SM, Skolil P, Salles JP, Dufouil C, Lehéricy S, Chupin M, Mangin JF, Bouhayia A, Allard M, Ricolfi F, Dubois D, Paule M, Martel B, Cotton F, Bonafé A, Chanalet S, Hugon F, Bonneville F, Cognard C, Chollet F, Payoux P, Voisin T, Peiffer S, Hitzel A, Allard M, Zanca M, Monteil J, Darcourt J, Molinier L, Derumeaux H, Costa N, Vincent C, Perret B, Vinel C, Olivier-Abbal P. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16(5):377–389. doi: 10.1016/S1474-4422(17)30040-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

