Ixekizumab improves sleep and work productivity in patients with non-radiographic axial spondyloarthritis: results from the COAST-X trial at 52 weeks
- PMID: 34560906
- PMCID: PMC8464085
- DOI: 10.1186/s41927-021-00218-y
Ixekizumab improves sleep and work productivity in patients with non-radiographic axial spondyloarthritis: results from the COAST-X trial at 52 weeks
Abstract
Background: Patients with non-radiographic axial spondyloarthritis experience negative impacts on sleep, work productivity, and activity impairment. Ixekizumab, a monoclonal antibody selectively targeting interleukin-17A, has shown efficacy in treating the signs and symptoms of non-radiographic axial spondyloarthritis. This analysis evaluated the effect of ixekizumab treatment on sleep, work productivity, and activity impairment in patients with non-radiographic axial spondyloarthritis.
Methods: COAST-X ( NCT02757352 ) was a 52-week, phase 3, multicenter, randomised placebo-controlled trial evaluating 80-mg ixekizumab every 2 weeks and every 4 weeks in patients with active non-radiographic axial spondyloarthritis. Sleep disturbance was measured with the Jenkins Sleep Evaluation Questionnaire (JSEQ) and analysed using mixed-effects models for repeated measures. Work productivity and activity impairment were measured using the Work Productivity and Activity Impairment Questionnaire for Spondyloarthritis and analysed using analysis of covariance. Absenteeism, presenteeism, and overall work impairment were assessed for patients reporting paid work; activity impairment was assessed regardless of work status.
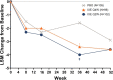
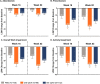
Results: Overall, patients treated with both dosing regimens of ixekizumab reported numerically greater improvements in sleep than placebo through Week 52. At Weeks 16 and 52, patients treated with ixekizumab every 4 weeks had significantly greater improvements in presenteeism (p = 0.007 and p = 0.003, respectively) and overall work impairment (p = 0.014 and p = 0.005, respectively) and numeric improvements in absenteeism than placebo. Patients treated with ixekizumab every 2 weeks had numerically greater improvements in absenteeism, presenteeism, and overall work impairment than placebo. Both dosing regimens of ixekizumab were associated with significantly greater improvements in activity impairment than placebo (ixekizumab every 4 weeks: p = 0.003 at Week 16 and p = 0.004 at Week 52; ixekizumab every 2 weeks: p = 0.007 at Week 16 and p = 0.006 at Week 52).
Conclusions: Treatment with ixekizumab improved sleep, work productivity, and activity impairment in patients with nr-axSpA. Improvements in presenteeism and overall work impairment were sustained and consistent in the patients treated with ixekizumab every 4 weeks from Week 16 to Week 52. Improvements in activity impairment were sustained and consistent in both ixekizumab-treated groups from Week 16 to Week 52.
Trial registration: NCT02757352 , May 2, 2016.
Keywords: Activity impairment; Ixekizumab; Non-radiographic axial spondyloarthritis; Sleep; Work productivity.
© 2021. The Author(s).
Conflict of interest statement
AD has received consulting fees from and has served on the advisory boards of AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Galapagos, Glaxo Smith & Kline, Janssen, Novartis, Pfizer, and UCB as well as receiving research grants from AbbVie, Eli Lilly, Glaxo Smith & Kline, Novartis, Pfizer, and UCB. PM has received research grants, consulting fees, and/or speaker fees from Abbvie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Galapagos, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, SUN Pharma, and UCB. HMO has received grant or research support from Janssen and Novartis, has received consulting fees from AbbVie, Celgene, Janssen, Eli Lilly and Company, Novartis, Pfizer, and UCB, and has received speaker fees from AbbVie, Biogen, Celgene, Janssen, Eli Lilly and Company, Novartis, Pfizer, Takeda, and UCB. TH, DS, AK, and SL are employees and shareholders of Eli Lilly and Company. AL is an employee of Syneos Health, vendor of Eli Lilly and Company. Victoria Navarro-Compán has consulting fees, speaker fees, and research grants from Abbvie, Bristol Myers Squibb, Janssen, Eli Lilly and Company, MSD, Novartis, Pfizer, Roche, and UCB.
Figures
References
-
- Deodhar A, van der Heijde D, Gensler LS, Kim TH, Maksymowych WP, Ostergaard M, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet (London, England) 2020;395(10217):53–64. doi: 10.1016/S0140-6736(19)32971-X. - DOI - PubMed
-
- Burgos-Varga R, Wei JC, Rahman MU, Akkoc N, Haq SA, Hammoudeh M, et al. The prevalence and clinical characteristics of nonradiographic axial spondyloarthritis among patients with inflammatory back pain in rheumatology practices: a multinational, multicenter study. Arthritis research & therapy. 2016;18(1):132. doi: 10.1186/s13075-016-1027-9. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

