Short-acting β2-agonist prescriptions are associated with poor clinical outcomes of asthma: the multi-country, cross-sectional SABINA III study
- PMID: 34561293
- PMCID: PMC9068976
- DOI: 10.1183/13993003.01402-2021
Short-acting β2-agonist prescriptions are associated with poor clinical outcomes of asthma: the multi-country, cross-sectional SABINA III study
Abstract
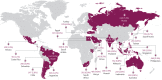
Background: To gain a global perspective on short-acting β2-agonist (SABA) prescriptions and associated asthma-related clinical outcomes in patients with asthma, we assessed primary health data across 24 countries in five continents.
Methods: SABINA III was a cross-sectional study that employed electronic case report forms at a study visit (in primary or specialist care) to record prescribed medication(s), over-the-counter (OTC) SABA purchases and clinical outcomes in asthma patients (≥12 years old) during the past 12 months. In patients with ≥1 SABA prescriptions, associations of SABA with asthma symptom control and severe exacerbations were analysed using multivariable regression models.
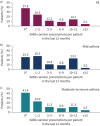
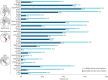
Results: Of 8351 patients recruited (n=6872, specialists; n=1440, primary care), 76.5% had moderate-to-severe asthma and 45.4% experienced ≥1 severe exacerbations in the past 12 months. 38% of patients were prescribed ≥3 SABA canisters; 18.0% purchased OTC SABA, of whom 76.8% also received SABA prescriptions. Prescriptions of 3-5, 6-9, 10-12 and ≥13 SABA canisters (versus 1-2) were associated with increasingly lower odds of controlled or partly controlled asthma (adjusted OR 0.64 (95% CI 0.53-0.78), 0.49 (95% CI 0.39-0.61), 0.42 (95% CI 0.34-0.51) and 0.33 (95% CI 0.25-0.45), respectively; n=4597) and higher severe exacerbation rates (adjusted incidence rate ratio 1.40 (95% CI 1.24-1.58), 1.52 (95% CI 1.33-1.74), 1.78 (95% CI 1.57-2.02) and 1.92 (95% CI 1.61-2.29), respectively; n=4612).
Conclusions: This study indicates an association between high SABA prescriptions and poor clinical outcomes across a broad range of countries, healthcare settings and asthma severities, providing support for initiatives to improve asthma morbidity by reducing SABA overreliance.
Trial registration: ClinicalTrials.gov NCT03857178.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: E.D. Bateman is a member of the Science Committee and Board of GINA, and reports personal fees from ALK, AstraZeneca, Boehringer Ingelheim, Chiesi, Menarini, Novartis, Orion, Regeneron Pharmaceuticals and Sanofi Genzyme. Conflict of interest: D.B. Price has board membership with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Regeneron, Sanofi Genzyme, Teva Pharmaceuticals and Thermo Fisher; consultancy agreements with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Novartis, Pfizer, Teva and Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Pfizer, Regeneron, Respiratory Effectiveness Group, Sanofi Genzyme, Teva, Theravance and the UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron, Sanofi Genzyme and Teva; payment for the development of educational materials from Mundipharma and Novartis; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis and Thermo Fisher; funding for patient enrolment or completion of research from Novartis; stock/stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); is a peer reviewer for grant committees of the Efficacy and Mechanism Evaluation programme and Health Technology Assessment; and was an expert witness for GlaxoSmithKline. Conflict of interest: H-C. Wang has nothing to disclose. Conflict of interest: A. Khattab has nothing to disclose. Conflict of interest: P. Schonffeldt reports lectures on medical education and inclusion as a researcher on clinical study protocols funded by AstraZeneca, GlaxoSmithKline, Teva, ITF Labomed, Boehringer Ingelheim and Sanofi Genzyme. Conflict of interest: A. Catanzariti is an employee of AstraZeneca. Conflict of interest: R.J.P. van der Valk is an employee of AstraZeneca and has shares in GlaxoSmithKline and shares and options in AstraZeneca. Conflict of interest: M.J.H.I. Beekman was an employee of AstraZeneca at the time the study was conducted and has shares in AstraZeneca.
Figures


Comment in
-
Cause or consequence?Eur Respir J. 2022 Jun 9;59(6):2103107. doi: 10.1183/13993003.03107-2021. Print 2022 Jun. Eur Respir J. 2022. PMID: 35210318 No abstract available.
-
Reply to: Cause or consequence?Eur Respir J. 2022 Jun 9;59(6):2200103. doi: 10.1183/13993003.00103-2022. Print 2022 Jun. Eur Respir J. 2022. PMID: 35210324 Free PMC article.
References
-
- Global Asthma Network . The Global Asthma Report 2018. 2018. Date last accessed: 12 May 2021. http://globalasthmareport.org/resources/Global_Asthma_Report_2018.pdf
-
- Royal College of Physicians . Why Asthma Still Kills: the National Review of Asthma Deaths (NRAD) Confidential Enquiry Report 2014. 2015. Date last accessed: 12 May 2021. www.rcplondon.ac.uk/projects/outputs/why-asthma-still-kills
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
