Edge current and pairing order transition in chiral bacterial vortices
- PMID: 34561308
- PMCID: PMC8488682
- DOI: 10.1073/pnas.2107461118
Edge current and pairing order transition in chiral bacterial vortices
Abstract
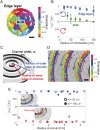
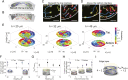
Bacterial suspensions show turbulence-like spatiotemporal dynamics and vortices moving irregularly inside the suspensions. Understanding these ordered vortices is an ongoing challenge in active matter physics, and their application to the control of autonomous material transport will provide significant development in microfluidics. Despite the extensive studies, one of the key aspects of bacterial propulsion has remained elusive: The motion of bacteria is chiral, i.e., it breaks mirror symmetry. Therefore, the mechanism of control of macroscopic active turbulence by microscopic chirality is still poorly understood. Here, we report the selective stabilization of chiral rotational direction of bacterial vortices in achiral circular microwells sealed by an oil/water interface. The intrinsic chirality of bacterial swimming near the top and bottom interfaces generates chiral collective motions of bacteria at the lateral boundary of the microwell that are opposite in directions. These edge currents grow stronger as bacterial density increases, and, within different top and bottom interfaces, their competition leads to a global rotation of the bacterial suspension in a favored direction, breaking the mirror symmetry of the system. We further demonstrate that chiral edge current favors corotational configurations of interacting vortices, enhancing their ordering. The intrinsic chirality of bacteria is a key feature of the pairing order transition from active turbulence, and the geometric rule of pairing order transition may shed light on the strategy for designing chiral active matter.
Keywords: bacterial vortex; chiral active matter; collective motion; microdevices; vortex pairing.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Zhang J., Libchaber A., Periodic boundary motion in thermal turbulence. Phys. Rev. Lett. 84, 4361–4364 (2000). - PubMed
-
- Ramaswamy S., The mechanics and statistics of active matter. Annu. Rev. Condens. Matter Phys. 1, 323–345 (2010).
-
- Marchetti M. C., et al. ., Hydrodynamics of soft active matter. Rev. Mod. Phys. 85, 1143 (2013).
-
- Alert R., Joanny J.-F., Casademunt J., Universal scaling of active nematic turbulence. Nat. Phys. 16, 682–688 (2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

