Antibody-dependent cellular cytotoxicity response to SARS-CoV-2 in COVID-19 patients
- PMID: 34561414
- PMCID: PMC8463587
- DOI: 10.1038/s41392-021-00759-1
Antibody-dependent cellular cytotoxicity response to SARS-CoV-2 in COVID-19 patients
Abstract
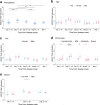
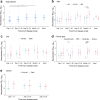
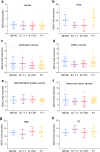
Antibody-dependent cellular cytotoxicity (ADCC) responses to viral infection are a form of antibody regulated immune responses mediated through the Fc fragment. Whether severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) triggered ADCC responses contributes to COVID-19 disease development is currently not well understood. To understand the potential correlation between ADCC responses and COVID-19 disease development, we analyzed the ADCC activity and neutralizing antibody response in 255 individuals ranging from asymptomatic to fatal infections over 1 year post disease. ADCC was elicited by 10 days post-infection, peaked by 11-20 days, and remained detectable until 400 days post-infection. In general, patients with severe disease had higher ADCC activities. Notably, patients who had severe disease and recovered had higher ADCC activities than patients who had severe disease and deceased. Importantly, ADCC activities were mediated by a diversity of epitopes in SARS-COV-2-infected mice and induced to comparable levels against SARS-CoV-2 variants of concern (VOCs) (B.1.1.7, B.1.351, and P.1) as that against the D614G mutant in human patients and vaccinated mice. Our study indicates anti-SARS-CoV-2 ADCC as a major trait of COVID-19 patients with various conditions, which can be applied to estimate the extra-neutralization level against COVID-19, especially lethal COVID-19.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

