Breaking a dative bond with mechanical forces
- PMID: 34561452
- PMCID: PMC8463581
- DOI: 10.1038/s41467-021-25932-6
Breaking a dative bond with mechanical forces
Abstract
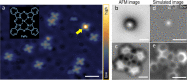
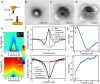
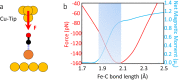
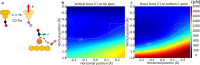
Bond breaking and forming are essential components of chemical reactions. Recently, the structure and formation of covalent bonds in single molecules have been studied by non-contact atomic force microscopy (AFM). Here, we report the details of a single dative bond breaking process using non-contact AFM. The dative bond between carbon monoxide and ferrous phthalocyanine was ruptured via mechanical forces applied by atomic force microscope tips; the process was quantitatively measured and characterized both experimentally and via quantum-based simulations. Our results show that the bond can be ruptured either by applying an attractive force of ~150 pN or by a repulsive force of ~220 pN with a significant contribution of shear forces, accompanied by changes of the spin state of the system. Our combined experimental and computational studies provide a deeper understanding of the chemical bond breaking process.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Giessibl FJ. High-speed force sensor for force microscopy and profilometry utilizing a quartz tuning fork. Appl. Phys. Lett. 1998;73:3956–3958. doi: 10.1063/1.122948. - DOI
-
- Schuler B, et al. Heavy oil based mixtures of different origins and treatments studied by atomic force microscopy. Energy Fuels. 2017;31:6856–6861. doi: 10.1021/acs.energyfuels.7b00805. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

