DREAMM-2: Indirect Comparisons of Belantamab Mafodotin vs. Selinexor + Dexamethasone and Standard of Care Treatments in Relapsed/Refractory Multiple Myeloma
- PMID: 34561812
- PMCID: PMC8523001
- DOI: 10.1007/s12325-021-01884-7
DREAMM-2: Indirect Comparisons of Belantamab Mafodotin vs. Selinexor + Dexamethasone and Standard of Care Treatments in Relapsed/Refractory Multiple Myeloma
Abstract
Introduction: Single-agent belantamab mafodotin (belamaf; BLENREP) demonstrated deep and durable responses in patients with relapsed/refractory multiple myeloma and ≥ 3 prior lines of therapy, including an immunomodulatory agent, proteasome inhibitor, and anti-CD38 antibody (DREAMM-2; NCT03525678).
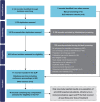
Methods: At the time of this study, STORM Part 2, NCT02336815 (selinexor plus low-dose dexamethasone; sel + dex) was systematically identified as the only feasible comparator to the DREAMM-2 cohort. Matching-adjusted indirect comparisons (MAIC) evaluated efficacy and safety of belamaf (2.5 mg/kg; n = 97) versus sel + dex (80 mg + 20 mg, respectively; n = 123). Populations were weighted for clinically validated effect modifiers and prognostic factors. Outcomes included overall survival (OS), progression-free survival (PFS), duration of response (DoR), overall response rate (ORR), time to response (TTR), and safety. The relative efficacy of belamaf versus standard of care (SoC) on OS was estimated by a Bucher indirect treatment comparison using the MAIC-adjusted hazard ratios (HR) for OS of belamaf (DREAMM-2) versus sel + dex (STORM Part 2) and a HR adjusted for refractoriness to carfilzomib and high-risk cytogenetics of sel + dex (STORM) versus SoC (MAMMOTH).
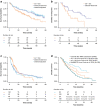
Results: Belamaf demonstrated improved OS (HR 0.53; 95% confidence interval 0.34, 0.83; p = 0.005) and DoR (0.41; 0.21, 0.83; p = 0.013) versus sel + dex. There were no statistically significant differences in ORR, TTR, and PFS. Belamaf had a favorable safety profile for most evaluable hematologic (any-grade, Grade 3-4) and non-hematologic (any-grade) adverse events versus sel + dex. Significantly improved OS was observed with belamaf versus SoC (0.29; 0.16, 0.54; p < 0.001).
Conclusion: Single-agent belamaf represents a new treatment option for triple-class refractory patients with RRMM.
Keywords: Belamaf; Indirect treatment comparison; MAIC; MAMMOTH; Matching-adjusted; RRMM; Selinexor; Survival.
© 2021. The Author(s).
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

