D-mannose alleviates osteoarthritis progression by inhibiting chondrocyte ferroptosis in a HIF-2α-dependent manner
- PMID: 34561933
- PMCID: PMC8560605
- DOI: 10.1111/cpr.13134
D-mannose alleviates osteoarthritis progression by inhibiting chondrocyte ferroptosis in a HIF-2α-dependent manner
Abstract
Objectives: Chondrocyte ferroptosis contributes to osteoarthritis (OA) progression, and D-mannose shows therapeutic value in many inflammatory conditions. Here, we investigated whether D-mannose interferes in chondrocyte ferroptotic cell death during osteoarthritic cartilage degeneration.
Materials and methods: In vivo anterior cruciate ligament transection (ACLT)-induced OA mouse model and an in vitro study of chondrocytes in an OA microenvironment induced by interleukin-1β (IL-1β) exposure were employed. Combined with Epas1 gene gain- and loss-of-function, histology, immunofluorescence, quantitative RT-PCR, Western blot, cell viability and flow cytometry experiments were performed to evaluate the chondroprotective effects of D-mannose in OA progression and the role of hypoxia-inducible factor 2 alpha (HIF-2 α) in D-mannose-induced ferroptosis resistance of chondrocytes.
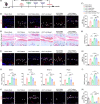
Results: D-mannose exerted a chondroprotective effect by attenuating the sensitivity of chondrocytes to ferroptosis and alleviated OA progression. HIF-2α was identified as a central mediator in D-mannose-induced ferroptosis resistance of chondrocytes. Furthermore, overexpression of HIF-2α in chondrocytes by Ad-Epas1 intra-articular injection abolished the chondroprotective effect of D-mannose during OA progression and eliminated the role of D-mannose as a ferroptosis suppressor.
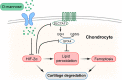
Conclusions: D-mannose alleviates osteoarthritis progression by suppressing HIF-2α-mediated chondrocyte sensitivity to ferroptosis, indicating D-mannose to be a potential therapeutic strategy for ferroptosis-related diseases.
© 2021 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures
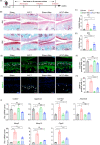

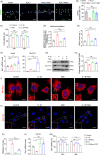
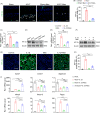
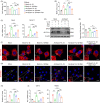
References
-
- Hunter DJ, Bierma‐Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745‐1759. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

