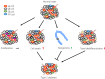
Diversity of pathophysiology in type 2 diabetes shown by islet pathology
- PMID: 34562302
- PMCID: PMC8756316
- DOI: 10.1111/jdi.13679
Diversity of pathophysiology in type 2 diabetes shown by islet pathology
Abstract
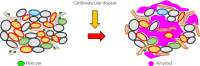
The etiology of type 2 diabetes is multifactorial, in which environmental and genetic factors are involved to varying degrees. This suggests that its pathophysiology might vary depending on the individuals. Knowledge of the differences is critical, because these differences are directly linked to the care and treatment of the patients. Recent studies have attempted to carry out subclassifications of type 2 diabetes based on clinical and genetic differences. However, there is no pathological evidence to support these subclassifications. The pathophysiology of type 2 diabetes is generally divided into insulin resistance in peripheral tissues and pancreatic islet dysfunction. Among them, islet dysfunction causes a deficit in insulin secretion from β-cells. In particular, a deficit in insulin secretion is ascribed to a combination of disruption of the insulin secretory machinery and a decrease in β-cell volume in type 2 diabetes. Recent research has suggested that transdifferentiation and dedifferentiation are involved in the decrease in β-cell volume, and that it might change dynamically depending on the glucose metabolic state. However, it is possible that the numbers of islet cells are decreased in type 2 diabetes. In particular, the loss of endocrine cells due to islet amyloid deposits is an important pathological change in type 2 diabetes in humans. These results show that pathological changes of the islets can be different in each individuals with type 2 diabetes and reflect each pathophysiology, which is useful in establishing further subclassifications and developing tailor-made therapies for type 2 diabetes.
Keywords: Amyloid; Islet pathology; Type 2 diabetes.
© 2021 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures



References
-
- De Fronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM: a balanced overview. Diabetes Care 1992; 15: 318–368. - PubMed
-
- Fukushima M, Usami M, Ikeda M, et al. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross‐sectional study of Japanese type 2 diabetes. Metabolism 2004; 53: 831–835. - PubMed
-
- Møller JB, Pedersen M, Tanaka H, et al. Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care 2014; 37: 796–804. - PubMed
-
- Kou K, Saisho Y, Satoh S, et al. Change in β‐cell mass in Japanese nondiabetic obese individuals. J Clin Endocrinol Metab 2013; 98: 3724–3730. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

