Generation of neutrophil extracellular traps in patients with acute liver failure is associated with poor outcome
- PMID: 34562318
- PMCID: PMC9299791
- DOI: 10.1002/hep.32174
Generation of neutrophil extracellular traps in patients with acute liver failure is associated with poor outcome
Abstract
Background and aims: Acute liver failure (ALF) is characterized by significant changes in the hemostatic system and by systemic inflammation. The formation of neutrophil extracellular traps (NETs), in which an activated neutrophil expels its DNA, histones, and granular enzymes, such as myeloperoxidase (MPO), has been associated with immune-mediated and thrombotic diseases. We hypothesized that formation of NETs in patients with ALF contributes to progression of disease.
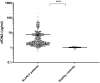
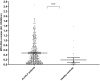
Approach and results: A total of 676 patients with ALF (international normalized ratio [INR], ≥1.5) or severe acute liver injury (ALI; INR, ≥2.0) were recruited from the U.S. ALF Study Group Registry between 2011 and 2018, of whom 308 patients (45.6%) had acetaminophen-induced ALF. Up to 21 days after admission, 483 patients (71.5%) survived without liver transplantation (LT). Levels of cell-free DNA (cfDNA) and the specific NET marker MPO-DNA complexes were measured in plasma samples obtained on admission and compared to levels in healthy controls. In addition, liver tissue obtained at transplantation of 20 ALF patients was stained for NETs. Levels of cfDNA were 7.1-fold, and MPO-DNA complexes 2.5-fold, higher in patients with ALF compared to healthy controls. cfDNA levels were not associated with 21-day transplant-free survival, but were higher in those patients with more-severe disease on admission, as reflected by various laboratory and clinical parameters. MPO-DNA levels were 30% higher in patients with ALF who died or required urgent LT. Liver tissue of ALF patients stained positive for NETs in 12 of 18 evaluable patients.
Conclusions: Here, we provide evidence for NET formation in patients with ALF. Elevated plasma levels of MPO-DNA complexes in patients with ALF were associated with poor outcome, which suggests that NET formation contributes to disease progression.
© 2021 The Authors. Hepatology published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Conflict of interest statement
Dr. Lee consults for and received grants from Gilead. He consults for Karuna, Forma, Cortexyme, Genentech, and SeaGen. He received grants from Intercept, Merck, Cumberland, Ocera, Alexion, Eiger, and Novo Nordisk.
Figures

Comment in
-
A NET gain in our understanding of acute liver failure.Hepatology. 2022 Mar;75(3):511-513. doi: 10.1002/hep.32278. Epub 2022 Jan 21. Hepatology. 2022. PMID: 34890053 No abstract available.
-
Letter to the editor: Etiology and coagulation should be considered more.Hepatology. 2022 Mar;75(3):769-770. doi: 10.1002/hep.32287. Epub 2022 Jan 5. Hepatology. 2022. PMID: 34923654 No abstract available.
-
Reply.Hepatology. 2022 Mar;75(3):770-771. doi: 10.1002/hep.32286. Epub 2022 Jan 5. Hepatology. 2022. PMID: 34923660 No abstract available.
References
-
- Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190–201. - PubMed
-
- Rolando N, Wade J, Davalos M, Wendon J, Philpott‐Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32(4 Pt. 1):734–9. - PubMed
-
- Lisman T, Stravitz RT. Rebalanced hemostasis in patients with acute liver failure. Semin Thromb Hemost. 2015;41:468–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

