The arbitrium system controls prophage induction
- PMID: 34562384
- PMCID: PMC8612738
- DOI: 10.1016/j.cub.2021.08.072
The arbitrium system controls prophage induction
Abstract
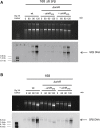
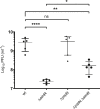
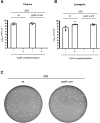
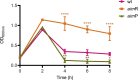
Some Bacillus-infecting bacteriophages use a peptide-based communication system, termed arbitrium, to coordinate the lysis-lysogeny decision. In this system, the phage produces AimP peptide during the lytic cycle. Once internalized by the host cell, AimP binds to the transcription factor AimR, reducing aimX expression and promoting lysogeny. Although these systems are present in a variety of mobile genetic elements, their role in the phage life cycle has only been characterized in phage phi3T during phage infection. Here, using the B. subtilis SPβ prophage, we show that the arbitrium system is also required for normal prophage induction. Deletion of the aimP gene increased phage reproduction, although the aimR deletion significantly reduced the number of phage particles produced after prophage induction. Moreover, our results indicated that AimR is involved in a complex network of regulation and brought forward two new players in the SPβ lysis-lysogeny decision system, YopN and the phage repressor YopR. Importantly, these proteins are encoded in an operon, the function of which is conserved across all SPβ-like phages encoding the arbitrium system. Finally, we obtained mutant phages in the arbitrium system, which behaved almost identically to the wild-type (WT) phage, indicating that the arbitrium system is not essential in the laboratory but is likely beneficial for phage fitness in nature. In support of this, by possessing a functional arbitrium system, the SPβ phage can optimize production of infective particles while also preserving the number of cells that survive after prophage induction, a strategy that increases phage persistence in nature.
Keywords: AimP; AimR; SOS response; SPβ phages; bacteriophage; lysis; lysis/lysogeny; lysogeny; phi3T; repressor.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Comment in
-
Decisions, decisions….Nat Rev Microbiol. 2022 Mar;20(3):125. doi: 10.1038/s41579-021-00677-7. Nat Rev Microbiol. 2022. PMID: 34931060 No abstract available.
References
-
- Corey L., Wald A., Celum C.L., Quinn T.C. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J. Acquir. Immune Defic. Syndr. 2004;35:435–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/V000772/1/MRC_/Medical Research Council/United Kingdom
- MR/S00940X/2/MRC_/Medical Research Council/United Kingdom
- BB/V002376/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/S00940X/1/MRC_/Medical Research Council/United Kingdom
- BB/N002873/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

