Regulation of prophage induction and lysogenization by phage communication systems
- PMID: 34562385
- PMCID: PMC8612742
- DOI: 10.1016/j.cub.2021.08.073
Regulation of prophage induction and lysogenization by phage communication systems
Abstract
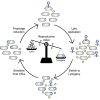
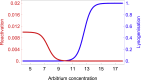
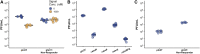
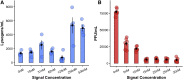
Many viruses cause both lytic infections, where they release viral particles, and dormant infections, where they await future opportunities to reactivate.1 The benefits of each transmission mode depend on the density of susceptible hosts in the environment.2-4 Some viruses infecting bacteria use molecular signaling to respond plastically to changes in host availability.5 These viruses produce a signal during lytic infection and regulate, based on the signal concentration in the environment, the probability with which they switch to causing dormant infections.5,6 We present an analytical framework to examine the adaptive significance of plasticity in viral life-history traits in fluctuating environments. Our model generalizes and extends previous theory7 and predicts that host density fluctuations should select for plasticity in entering lysogeny as well as virus reactivation once signal concentrations decline. Using Bacillus subtilis and its phage phi3T, we experimentally confirm the prediction that phages use signal to make informed decisions over prophage induction. We also demonstrate that lysogens produce signaling molecules and that signal is degraded by hosts in a density-dependent manner. Declining signal concentrations therefore potentially indicate the presence of uninfected hosts and trigger prophage induction. Finally, we find that conflict over the responses of lysogenization and reactivation to signal is resolved through the evolution of different response thresholds for each trait. Collectively, these findings deepen our understanding of the ways viruses use molecular communication to regulate their infection strategies, which can be leveraged to manipulate host and phage population dynamics in natural environments.
Keywords: arbitrium; induction; lysis-lysogeny; microbiology; phage; prophage.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

Comment in
-
Decisions, decisions….Nat Rev Microbiol. 2022 Mar;20(3):125. doi: 10.1038/s41579-021-00677-7. Nat Rev Microbiol. 2022. PMID: 34931060 No abstract available.
References
-
- Gandon S. Why be temperate: lessons from bacteriophage λ. Trends Microbiol. 2016;24:356–365. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

