Vitamin D signaling inhibits HBV activity by directly targeting the HBV core promoter
- PMID: 34562448
- PMCID: PMC8517215
- DOI: 10.1016/j.jbc.2021.101233
Vitamin D signaling inhibits HBV activity by directly targeting the HBV core promoter
Abstract
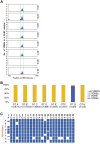
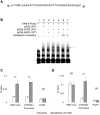
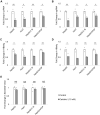
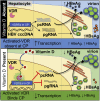
Clinical and epidemiological studies support a role for vitamin D in suppressing hepatitis B virus (HBV). This antiviral role of vitamin D is widely attributed to vitamin D receptor (VDR)/retinoid X receptor-mediated regulation of host immunomodulatory genes through vitamin D response elements (VDREs) in their promoters. Here, we investigated the ability of calcitriol (1α,25-dihydroxyvitamin D3, metabolically activated vitamin D) to directly regulate HBV activity through this signaling pathway. We observed that calcitriol selectively inhibited only the HBV core promoter without affecting the HBV-PreS1, HBV-PreS2/S, or HBx promoters. We then identified a VDRE cluster in the HBV core promoter that is highly conserved across most HBV genotypes. Disruption of this VDRE cluster abrogated calcitriol-mediated suppression of the HBV core promoter. Furthermore, we showed that VDR interacts directly with the VDRE cluster in the HBV core promoter independent of retinoid X receptor. This demonstrates that calcitriol inhibits HBV core promoter activity through a noncanonical calcitriol-activated VDR pathway. Finally, we observed that calcitriol suppressed expression of the canonical HBV core promoter transcripts, pregenomic RNA, and precore RNA in multiple HBV cell culture models. In addition, calcitriol inhibited the secretion of hepatitis B "e" antigen and hepatitis B surface antigen (HBV-encoded proteins linked to poor disease prognosis), without affecting virion secretion. Our findings identify VDR as a novel regulator of HBV core promoter activity and also explain at least in part the correlation of vitamin D levels to HBV activity observed in clinical studies. Furthermore, this study has implications on the potential use of vitamin D along with anti-HBV therapies, and lays the groundwork for studies on vitamin D-mediated regulation of viruses through VDREs in virus promoters.
Keywords: HBV core promoter; antiviral agent; cell signaling; gene regulation; hepatitis B virus; nuclear receptor; viral transcription; vitamin D; vitamin D receptor; vitamin D response element.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

