Polypeptide Composition and Topology Affect Hydrogelation of Star-Shaped Poly(L-lysine)-Based Amphiphilic Copolypeptides
- PMID: 34563017
- PMCID: PMC8482192
- DOI: 10.3390/gels7030131
Polypeptide Composition and Topology Affect Hydrogelation of Star-Shaped Poly(L-lysine)-Based Amphiphilic Copolypeptides
Abstract
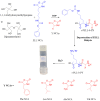
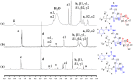
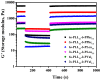
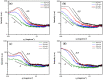
In this research, we studied the effect of polypeptide composition and topology on the hydrogelation of star-shaped block copolypeptides based on hydrophilic, coil poly(L-lysine)20 (s-PLL20) tethered with a hydrophobic, sheet-like polypeptide segment, which is poly(L-phenylalanine) (PPhe), poly(L-leucine) (PLeu), poly(L-valine) (PVal) or poly(L-alanine) (PAla) with a degree of polymerization (DP) about 5. We found that the PPhe, PLeu, and PVal segments are good hydrogelators to promote hydrogelation. The hydrogelation and hydrogel mechanical properties depend on the arm number and hydrophobic polypeptide segment, which are dictated by the amphiphilic balance between polypeptide blocks and the hydrophobic interactions/hydrogen bonding exerted by the hydrophobic polypeptide segment. The star-shaped topology could facilitate their hydrogelation due to the branching chains serving as multiple interacting depots between hydrophobic polypeptide segments. The 6-armed diblock copolypeptides have better hydrogelation ability than 3-armed ones and s-PLL-b-PPhe exhibits better hydrogelation ability than s-PLL-b-PVal and s-PLL-b-PLeu due to the additional cation-π and π-π interactions. This study highlights that polypeptide composition and topology could be additional parameters to manipulate polypeptide hydrogelation.
Keywords: chain conformation; hydrogels; polymer topology; polypeptide; self-assembly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

