Polyphenols-loaded electrospun nanofibers in bone tissue engineering and regeneration
- PMID: 34563260
- PMCID: PMC8466400
- DOI: 10.1186/s40824-021-00229-3
Polyphenols-loaded electrospun nanofibers in bone tissue engineering and regeneration
Abstract
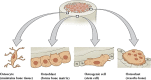
Bone is a complex structure with unique cellular and molecular process in its formation. Bone tissue regeneration is a well-organized and routine process at the cellular and molecular level in humans through the activation of biochemical pathways and protein expression. Though many forms of biomaterials have been applied for bone tissue regeneration, electrospun nanofibrous scaffolds have attracted more attention among researchers with their physicochemical properties such as tensile strength, porosity, and biocompatibility. When drugs, antibiotics, or functional nanoparticles are taken as additives to the nanofiber, its efficacy towards the application gets increased. Polyphenol is a versatile green/phytochemical small molecule playing a vital role in several biomedical applications, including bone tissue regeneration. When polyphenols are incorporated as additives to the nanofibrous scaffold, their combined properties enhance cell attachment, proliferation, and differentiation in bone tissue defect. The present review describes bone biology encompassing the composition and function of bone tissue cells and exemplifies the series of biological processes associated with bone tissue regeneration. We have highlighted the molecular mechanism of bioactive polyphenols involved in bone tissue regeneration and specified the advantage of electrospun nanofiber as a wound healing scaffold. As the polyphenols contribute to wound healing with their antioxidant and antimicrobial properties, we have compiled a list of polyphenols studied, thus far, for bone tissue regeneration along with their in vitro and in vivo experimental biological results and salient observations. Finally, we have elaborated on the importance of polyphenol-loaded electrospun nanofiber in bone tissue regeneration and discussed the possible challenges and future directions in this field.
Keywords: Bone tissue regeneration; Drug loading; Electrospun nanofiber; Polyphenols.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

