Amplification of neurotoxic HTTex1 assemblies in human neurons
- PMID: 34563643
- PMCID: PMC8943833
- DOI: 10.1016/j.nbd.2021.105517
Amplification of neurotoxic HTTex1 assemblies in human neurons
Abstract
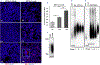
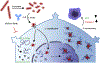
Huntington's disease (HD) is a genetically inherited neurodegenerative disorder caused by expansion of a polyglutamine (polyQ) repeat in the exon-1 of huntingtin protein (HTT). The expanded polyQ enhances the amyloidogenic propensity of HTT exon 1 (HTTex1), which forms a heterogeneous mixture of assemblies with a broad neurotoxicity spectrum. While predominantly intracellular, monomeric and aggregated mutant HTT species are also present in the cerebrospinal fluids of HD patients, however, their biological properties are not well understood. To explore the role of extracellular mutant HTT in aggregation and toxicity, we investigated the uptake and amplification of recombinant HTTex1 assemblies in cell culture models. We find that small HTTex1 fibrils preferentially enter human neurons and trigger the amplification of neurotoxic assemblies; astrocytes or epithelial cells are not permissive. The amplification of HTTex1 in neurons depletes endogenous HTT protein with non-pathogenic polyQ repeat, activates apoptotic caspase-3 pathway and induces nuclear fragmentation. Using a panel of novel monoclonal antibodies and genetic mutation, we identified epitopes within the N-terminal 17 amino acids and proline-rich domain of HTTex1 to be critical in neural uptake and amplification. Synaptosome preparations from the brain homogenates of HD mice also contain mutant HTT species, which enter neurons and behave similar to small recombinant HTTex1 fibrils. These studies suggest that amyloidogenic extracellular mutant HTTex1 assemblies may preferentially enter neurons, propagate and promote neurodegeneration.
Keywords: Huntingtin; Huntingtin exon1; Huntington's disease; Neurotoxicity; Seeding.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest
Authors declare no competing interest.
Figures


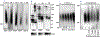


References
-
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S, 2004. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 431, 805–810. - PubMed
-
- Ast A, et al., 2018. mHTT Seeding Activity: A Marker of Disease Progression and Neurotoxicity in Models of Huntington’s Disease. Mol. Cell 71, 675–688. - PubMed
-
- Bates GP, et al., 2015. Huntington disease. Nat. Rev. Dis. Primers 1, 15005. - PubMed
-
- Bäuerlein FJB, et al., 2017. In Situ Architecture and Cellular Interactions of PolyQ Inclusions. Cell. 171, 179–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

