Genome edited B cells: a new frontier in immune cell therapies
- PMID: 34563675
- PMCID: PMC8571172
- DOI: 10.1016/j.ymthe.2021.09.019
Genome edited B cells: a new frontier in immune cell therapies
Abstract
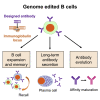
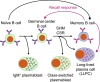
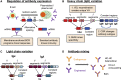
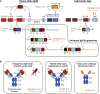
Cell therapies based on reprogrammed adaptive immune cells have great potential as "living drugs." As first demonstrated clinically for engineered chimeric antigen receptor (CAR) T cells, the ability of such cells to undergo clonal expansion in response to an antigen promotes both self-renewal and self-regulation in vivo. B cells also have the potential to be developed as immune cell therapies, but engineering their specificity and functionality is more challenging than for T cells. In part, this is due to the complexity of the immunoglobulin (Ig) locus, as well as the requirement for regulated expression of both cell surface B cell receptor and secreted antibody isoforms, in order to fully recapitulate the features of natural antibody production. Recent advances in genome editing are now allowing reprogramming of B cells by site-specific engineering of the Ig locus with preformed antibodies. In this review, we discuss the potential of engineered B cells as a cell therapy, the challenges involved in editing the Ig locus and the advances that are making this possible, and envision future directions for this emerging field of immune cell engineering.
Keywords: B cells; Cas9; HIV; cell therapy; genome editing; immunotherapy.
Copyright © 2021 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

