Marine Pyrrole Alkaloids
- PMID: 34564176
- PMCID: PMC8471394
- DOI: 10.3390/md19090514
Marine Pyrrole Alkaloids
Abstract
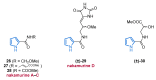
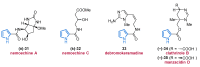
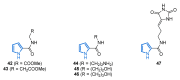
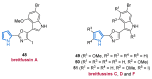
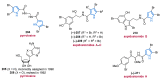
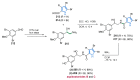
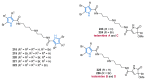
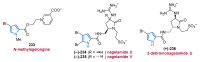
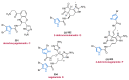
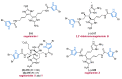
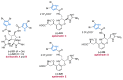
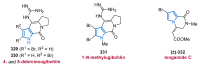
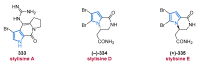
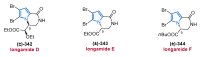
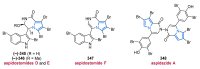
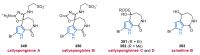
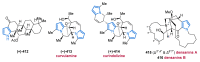
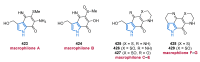
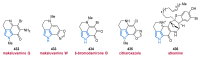
Nitrogen heterocycles are essential parts of the chemical machinery of life and often reveal intriguing structures. They are not only widespread in terrestrial habitats but can also frequently be found as natural products in the marine environment. This review highlights the important class of marine pyrrole alkaloids, well-known for their diverse biological activities. A broad overview of the marine pyrrole alkaloids with a focus on their isolation, biological activities, chemical synthesis, and derivatization covering the decade from 2010 to 2020 is provided. With relevant structural subclasses categorized, this review shall provide a clear and timely synopsis of this area.
Keywords: alkaloids; bromopyrroles; marine natural products; nitrogen heterocycles; pyrrole-aminoimidazole alkaloids; pyrrole-imidazole alkaloids; pyrroles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
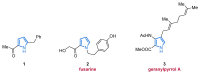

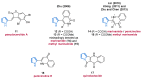
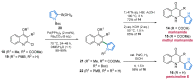
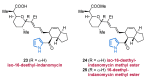


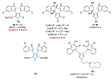

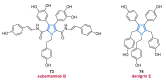
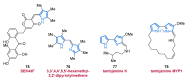
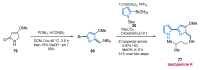
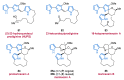


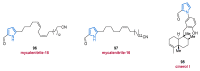
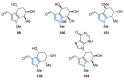
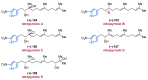







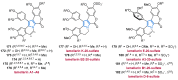
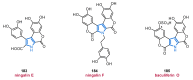

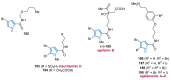
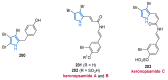













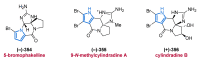
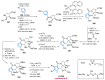
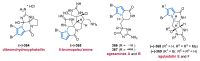
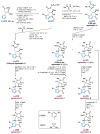
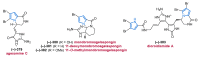
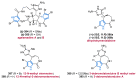

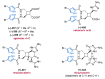

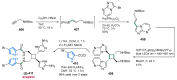

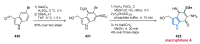

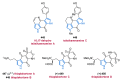

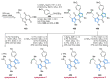
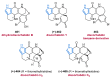
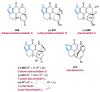
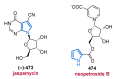
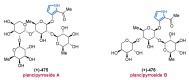
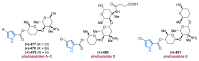
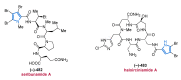
References
-
- Tasdemir D. Biodiversität im Meer und an Land. Vom Wert Biologischer Vielfalt. Deutsches GeoForschungsZentrum GFZ; Potsdam, Germany: 2020. Naturstoffe aus dem Meer für Medizin und Landwirtschaft; pp. 47–49.
-
- Blessie E.J., Wruck W., Abbey B.A., Ncube A., Graffmann N., Amarh V., Arthur P.A., Adjaye J. Transcriptomic Analysis of Marine Endophytic Fungi Extract Identifies Highly Enriched Anti-Fungal Fractions Targeting Cancer Pathways in HepG2 Cell Lines. BMC Genom. 2020;21:265. doi: 10.1186/s12864-020-6684-z. - DOI - PMC - PubMed
-
- Delgado-Roche L., González K., Mesta F., Couder B., Tavarez Z., Zavala R., Hernandez I., Garrido G., Rodeiro I., Vanden Berghe W. Polyphenolic Fraction Obtained from Thalassia testudinum Marine Plant and Thalassiolin B Exert Cytotoxic Effects in Colorectal Cancer Cells and Arrest Tumor Progression in a Xenograft Mouse Model. Front. Pharmacol. 2020;11:592985. doi: 10.3389/fphar.2020.592985. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

