Extraction Procedure, Characteristics, and Feasibility of Caulerpa microphysa (Chlorophyta) Polysaccharide Extract as a Cosmetic Ingredient
- PMID: 34564186
- PMCID: PMC8470774
- DOI: 10.3390/md19090524
Extraction Procedure, Characteristics, and Feasibility of Caulerpa microphysa (Chlorophyta) Polysaccharide Extract as a Cosmetic Ingredient
Abstract
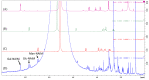
The green alga Caulerpa microphysa, which is native to Taiwan, has a relatively high economic value and a well-developed culture technique, and is used mainly as a foodstuff. Its extract has been shown to exhibit antitumor properties, but the polysaccharide content of the extract and its anti-inflammatory and wound-healing effects and moisture-absorption and -retention capacity remain unknown. Hence, the objective of this study was to evaluate the potential of the polysaccharides in C. microphysa extract (CME) for use in cosmetics. The overall polysaccharide yield from the CME was 73.93% w/w, with four molecular weight fractions. The polysaccharides comprised 59.36 mol% mannose, 27.16 mol% glucose, and 13.48 mol% galactose. In addition, the CME exhibited strong antiallergic, wound-healing, transdermal-delivery, and moisture-absorption and -retention effects. In conclusion, the results suggested that CME potentially has anti-inflammatory and wound-healing effects and a good moisture capacity, which can be used in cosmetic applications.
Keywords: Caulerpa; anti-inflammation; moisture capacity; polysaccharides; wound healing.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that may have influenced the work reported in this paper.
Figures








References
-
- Guidara M., Yaich H., Amor I.B., Fakhfakh J., Gargouri J., Lassoued S., Blecker C., Richel A., Attia H., Garna H. Effect of extraction procedures on the chemical structure, antitumor and anticoagulant properties of ulvan from Ulva lactuca of Tunisia coast. Carbohydr. Polym. 2021;253:117283. doi: 10.1016/j.carbpol.2020.117283. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

