Nontargeted Metabolomics as a Screening Tool for Estimating Bioactive Metabolites in the Extracts of 50 Indigenous Korean Plants
- PMID: 34564401
- PMCID: PMC8468114
- DOI: 10.3390/metabo11090585
Nontargeted Metabolomics as a Screening Tool for Estimating Bioactive Metabolites in the Extracts of 50 Indigenous Korean Plants
Abstract
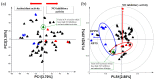
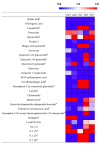
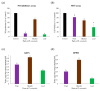
Many indigenous Korean plants have been used in medicinal preparations and health-promoting foods. These plant species contain beneficial metabolites with various bioactivities, such as antioxidant and anti-inflammatory activities. Herein, we suggest a new screening strategy using metabolomics to explore the bioactive compounds in 50 Korean plants. Secondary metabolites were analyzed using UHPLC-LTQ-Orbitrap-MS/MS. The plant extracts were subjected to antioxidant and anti-inflammatory assays. We identified metabolites that contributed to bioactivities according to the results of bioassays and multivariate analyses. Using Pearson's correlation, phenolics (e.g., casuarictin, 3-O-methylellagic acid) showed positive correlation with antioxidant activity, while biflavonoids (e.g., amentoflavone, rosbustaflavone) were correlated with nitric oxide (NO) inhibition activity. To compensate for the limitation of this new strategy, we further validated these by investigating three parts (branches, fruits, leaves) of Platycladus orientalis which showed high activities on both bioassays. Unlike the above observation, we identified significantly different metabolites from different parts, which was not the results of bioassays. In these validation steps, interestingly, biflavonoids (e.g., robustaflavone, sciadopitysin) contributed to both activities in P. orientalis. The findings of this work suggest that new strategy could be more beneficial in the identification of bioactive plant species as well as that of their corresponding bioactive compounds that impart the bioactivity.
Keywords: UHPLC-LTQ-Orbitrap-MS/MS; anti-inflammatory activity; antioxidant activity; indigenous plant; metabolite profiling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Choi C.W., Kim S.C., Hwang S.S., Choi B.K., Ahn H.J., Lee M.Y., Park S.H., Kim S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002;163:1161–1168. doi: 10.1016/S0168-9452(02)00332-1. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

