Metabolic Profiling during Acute Myeloid Leukemia Progression Using Paired Clinical Bone Marrow Serum Samples
- PMID: 34564403
- PMCID: PMC8471543
- DOI: 10.3390/metabo11090586
Metabolic Profiling during Acute Myeloid Leukemia Progression Using Paired Clinical Bone Marrow Serum Samples
Abstract
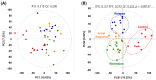
Cellular metabolic changes reflect the characteristics of patients with acute myeloid leukemia (AML) caused by genetic variations, which are important in establishing AML treatment. However, little is known about the metabolic profile of patients with genetic variation-induced AML. Furthermore, the metabolites differ with disease progression. Here, metabolites in the bone marrow serum of ten patients with AML and healthy individuals were analyzed using gas chromatography-mass spectrometry. Compared with that in healthy individuals, expression of most metabolites decreased in patients with AML; hydroxylamine, 2-hydroxybutyric acid, monomethylphosphate, and ethylphosphate expression was unusually increased in the patients. We further examined serial metabolite changes across the initial diagnosis, postremission, and relapse phases. Patients with relapse showed increased metabolite expression compared with those in the diagnostic phase, confirming that patients with AML had aggressively modified leukemic cells. However, a clear difference in metabolite distribution was not observed between the diagnosis and complete remission phases, suggesting that the metabolic microenvironment did not change significantly despite complete remission. Interestingly, metabolite profiles differed with genetic variations in leukemic cells. Our results, which were obtained using paired samples collected during AML progression, provide valuable insights for identifying vulnerable targets in the AML metabolome and developing new treatment strategies.
Keywords: acute myeloid leukemia; bone marrow serum; cell metabolism; metabolomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cancer Genome Atlas Research Network. Ley T.J., Miller C., Ding L., Raphael B.J., Mungall A.J., Robertson A., Hoadley K., Triche T.J., Laird P.W., et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. - DOI - PMC - PubMed
-
- Druker B.J., Guilhot F., O’Brien S.G., Gathmann I., Kantarjian H., Gattermann N., Deininger M.W., Silver R.T., Goldman J.M., Stone R.M., et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. - DOI - PubMed
LinkOut - more resources
Full Text Sources

