Transferrin Biosynthesized in the Brain Is a Novel Biomarker for Alzheimer's Disease
- PMID: 34564432
- PMCID: PMC8470343
- DOI: 10.3390/metabo11090616
Transferrin Biosynthesized in the Brain Is a Novel Biomarker for Alzheimer's Disease
Abstract
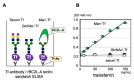
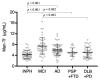
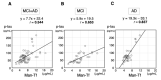
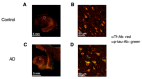
Glycosylation is a cell type-specific post-translational modification that can be used for biomarker identification in various diseases. Aim of this study is to explore glycan-biomarkers on transferrin (Tf) for Alzheimer's disease (AD) in cerebrospinal fluid (CSF). Glycan structures of CSF Tf were analyzed by ultra-performance liquid chromatography followed by mass spectrometry. We found that a unique mannosylated-glycan is carried by a Tf isoform in CSF (Man-Tf). The cerebral cortex contained Man-Tf as a major isofom, suggesting that CSF Man-Tf is, at least partly, derived from the cortex. Man-Tf levels were analyzed in CSF of patients with neurological diseases. Concentrations of Man-Tf were significantly increased in AD and mild cognitive impairment (MCI) comparing with other neurological diseases, and the levels correlated well with those of phosphorylated-tau (p-tau), a representative AD marker. Consistent with the observation, p-tau and Tf were co-expressed in hippocampal neurons of AD, leading to the notion that a combined p-tau and Man-Tf measure could be a biomarker for AD. Indeed, levels of p-tau x Man-Tf showed high diagnostic accuracy for MCI and AD; 84% sensitivities and 90% specificities for MCI and 94% sensitivities and 89% specificities for AD. Thus Man-Tf could be a new biomarker for AD.
Keywords: Alzheimer’s disease; biomarker; cerebrospinal fluid; glycan-isoforms; transferrin.
Conflict of interest statement
The authors have no potential conflicts of interest to report.
Figures






References
-
- Kalaria R.N., Maestre G.E., Arizaga R., Friedland R.P., Galasko D., Hall K., Luchsinger J.A., Ogunniyi A., Perry E.K., Potocnik F., et al. Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. - DOI - PMC - PubMed
-
- McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

