Homomeric and Heteromeric α7 Nicotinic Acetylcholine Receptors in Health and Some Central Nervous System Diseases
- PMID: 34564481
- PMCID: PMC8465519
- DOI: 10.3390/membranes11090664
Homomeric and Heteromeric α7 Nicotinic Acetylcholine Receptors in Health and Some Central Nervous System Diseases
Abstract
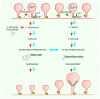
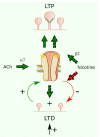
Nicotinic acetylcholine receptors (nAChRs) are pentameric ligand-gated ion channels involved in the modulation of essential brain functions such as memory, learning, and attention. Homomeric α7 nAChR, formed exclusively by five identical α7 subunits, is involved in rapid synaptic transmission, whereas the heteromeric oligomers composed of α7 in combination with β subunits display metabotropic properties and operate in slower time frames. At the cellular level, the activation of nAChRs allows the entry of Na+ and Ca2+; the two cations depolarize the membrane and trigger diverse cellular signals, depending on the type of nAChR pentamer and neurons involved, the location of the intervening cells, and the networks of which these neuronal cells form part. These features make the α7 nAChR a central player in neurotransmission, metabolically associated Ca2+-mediated signaling, and modulation of diverse fundamental processes operated by other neurotransmitters in the brain. Due to its ubiquitous distribution and the multiple functions it displays in the brain, the α7 nAChR is associated with a variety of neurological and neuropsychiatric disorders whose exact etiopathogenic mechanisms are still elusive.
Keywords: acetylcholine receptor; cholinergic neurotransmission; dendritic spine; neurotransmitter receptor protein; nicotinic; synaptopathy; α7 nAChR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

