Photocatalytic Nanofiber Membranes for the Degradation of Micropollutants and Their Antimicrobial Activity: Recent Advances and Future Prospects
- PMID: 34564496
- PMCID: PMC8467043
- DOI: 10.3390/membranes11090678
Photocatalytic Nanofiber Membranes for the Degradation of Micropollutants and Their Antimicrobial Activity: Recent Advances and Future Prospects
Abstract
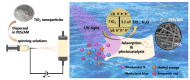
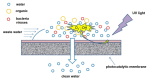
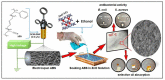
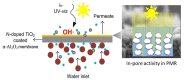
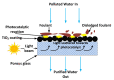
This review paper systematically evaluates current progress on the development and performance of photocatalytic nanofiber membranes often used in the removal of micropollutants from water systems. It is demonstrated that nanofiber membranes serve as excellent support materials for photocatalytic nanoparticles, leading to nanofiber membranes with enhanced optical properties, as well as improved recovery, recyclability, and reusability. The tremendous performance of photocatalytic membranes is attributed to the photogenerated reactive oxygen species such as hydroxyl radicals, singlet oxygen, and superoxide anion radicals introduced by catalytic nanoparticles such as TiO2 and ZnO upon light irradiation. Hydroxyl radicals are the most reactive species responsible for most of the photodegradation processes of these unwanted pollutants. The review also demonstrates that self-cleaning and antimicrobial nanofiber membranes are useful in the removal of microbial species in water. These unique materials are also applicable in other fields such as wound dressing since the membrane allows for oxygen flow in wounds to heal while antimicrobial agents protect wounds against infections. It is demonstrated that antimicrobial activities against bacteria and photocatalytic degradation of micropollutants significantly reduce membrane fouling. Therefore, the review demonstrates that electrospun photocatalytic nanofiber membranes with antimicrobial activity form efficient cost-effective multifunctional composite materials for the removal of unwanted species in water and for use in various other applications such as filtration, adsorption and electrocatalysis.
Keywords: antimicrobial properties; micropollutants; nanofiber membranes; photocatalysis; wastewater treatment.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures














References
-
- Bi O., Charles L., Casado C., Marugán J., Septien S., Ndlovu T., Nsikayezwe L. Photocatalytic degradation of atrazine in aqueous solution using hyperbranched polyethyleneimine templated morphologies of BiVO4 fused. J. Environ. Chem. Eng. 2020;8:104215. doi: 10.1016/j.jece.2020.104215. - DOI
-
- Nieto-delgado C., Partida-gutierrez D., Rangel-mendez J.R. Preparation of activated carbon cloths from renewable natural fabrics and their performance during the adsorption of model organic and inorganic pollutants in water. J. Clean. Prod. 2019;213:650–658. doi: 10.1016/j.jclepro.2018.12.184. - DOI
-
- Cai W., Yu J., Jaroniec M. Template-free synthesis of hierarchical spindle-like g-Al2O3 materials and their adsorption affinity towards organic and inorganic pollutants in water. J. Mater. Chem. 2010;20:4587–4594. doi: 10.1039/b924366f. - DOI
Publication types
LinkOut - more resources
Full Text Sources

