Genetic Diversity of Dengue Virus in Clinical Specimens from Bangkok, Thailand, during 2018-2020: Co-Circulation of All Four Serotypes with Multiple Genotypes and/or Clades
- PMID: 34564546
- PMCID: PMC8482112
- DOI: 10.3390/tropicalmed6030162
Genetic Diversity of Dengue Virus in Clinical Specimens from Bangkok, Thailand, during 2018-2020: Co-Circulation of All Four Serotypes with Multiple Genotypes and/or Clades
Abstract
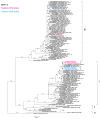
Dengue is an arboviral disease highly endemic in Bangkok, Thailand. To characterize the current genetic diversity of dengue virus (DENV), we recruited patients with suspected DENV infection at the Hospital for Tropical Diseases, Bangkok, during 2018-2020. We determined complete nucleotide sequences of the DENV envelope region for 111 of 276 participant serum samples. All four DENV serotypes were detected, with the highest proportion being DENV-1. Although all DENV-1 sequences were genotype I, our DENV-1 sequences were divided into four distinct clades with different distributions in Asian countries. Two genotypes of DENV-2 were identified, Asian I and Cosmopolitan, which were further divided into two and three distinct clades, respectively. In DENV-3, in addition to the previously dominant genotype III, a cluster of 6 genotype I viruses only rarely reported in Thailand was also observed. All of the DENV-4 viruses belonged to genotype I, but they were separated into three distinct clades. These results indicated that all four serotypes of DENV with multiple genotypes and/or clades co-circulate in Bangkok. Continuous investigation of DENV is warranted to further determine the relationship between DENV within Thailand and neighboring countries in Southeast Asia and Asia.
Keywords: Bangkok; DENVs; clades; co-circulation; genetic diversity; genotypes.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Emergence of genotype Cosmopolitan of dengue virus type 2 and genotype III of dengue virus type 3 in Thailand.PLoS One. 2018 Nov 12;13(11):e0207220. doi: 10.1371/journal.pone.0207220. eCollection 2018. PLoS One. 2018. PMID: 30419004 Free PMC article.
-
Phylogenetic analysis revealed the co-circulation of four dengue virus serotypes in Southern Thailand.PLoS One. 2019 Aug 15;14(8):e0221179. doi: 10.1371/journal.pone.0221179. eCollection 2019. PLoS One. 2019. PMID: 31415663 Free PMC article.
-
Genotype replacement of dengue virus type 3 and clade replacement of dengue virus type 2 genotype Cosmopolitan in Dhaka, Bangladesh in 2017.Infect Genet Evol. 2019 Nov;75:103977. doi: 10.1016/j.meegid.2019.103977. Epub 2019 Jul 24. Infect Genet Evol. 2019. PMID: 31351235
-
Dengue virus in humans and mosquitoes and their molecular characteristics in northeastern Thailand 2016-2018.PLoS One. 2021 Sep 14;16(9):e0257460. doi: 10.1371/journal.pone.0257460. eCollection 2021. PLoS One. 2021. PMID: 34520486 Free PMC article.
-
A Six Years (2010-2016) Longitudinal Survey of the Four Serotypes of Dengue Viruses in Lao PDR.Microorganisms. 2023 Jan 18;11(2):243. doi: 10.3390/microorganisms11020243. Microorganisms. 2023. PMID: 36838207 Free PMC article.
Cited by
-
Dynamics and genetic variation of dengue virus serotypes circulating during the 2022 outbreak in Karachi.Sci Rep. 2025 Jul 2;15(1):22703. doi: 10.1038/s41598-025-07606-1. Sci Rep. 2025. PMID: 40594640 Free PMC article.
-
Genomic Characterization of Circulating Dengue Virus, Ethiopia, 2022-2023.Emerg Infect Dis. 2025 Mar;31(3):516-525. doi: 10.3201/eid3103.240996. Emerg Infect Dis. 2025. PMID: 40023801 Free PMC article.
-
Diagnostic Performance of Dengue NS1 and Antibodies by Serum Concentration Technique.Trop Med Infect Dis. 2023 Feb 14;8(2):117. doi: 10.3390/tropicalmed8020117. Trop Med Infect Dis. 2023. PMID: 36828533 Free PMC article.
-
Unraveling Dengue Virus Diversity in Asia: An Epidemiological Study through Genetic Sequences and Phylogenetic Analysis.Viruses. 2024 Jun 28;16(7):1046. doi: 10.3390/v16071046. Viruses. 2024. PMID: 39066210 Free PMC article. Review.
-
The Spatiotemporal Distribution and Molecular Characterization of Circulating Dengue Virus Serotypes/Genotypes in Senegal from 2019 to 2023.Trop Med Infect Dis. 2024 Jan 27;9(2):32. doi: 10.3390/tropicalmed9020032. Trop Med Infect Dis. 2024. PMID: 38393121 Free PMC article.
References
-
- Okanurak K., Sornmani S., Indaratna K. The cost of dengue hemorrhagic fever in Thailand. Southeast Asian J. Trop. Med. Public Health. 1997;28:711–717. - PubMed
-
- World Health Organization . Global Strategy for Dengue Prevention and Control 2012–2020. World Health Organization; Geneva, Switzerland: 2012.
-
- Medeiros A.S., Costa D.M.P., Branco M.S.D., Sousa D.M.C., Monteiro J.D., Galvao S.P.M., Azevedo P.R.M., Fernandes J.V., Jeronimo S.M.B., Araujo J.M.G. Dengue virus in Aedes aegypti and Aedes albopictus in urban areas in the state of Rio Grande do Norte, Brazil: Importance of virological and entomological surveillance. PLoS ONE. 2018;13:e0194108. doi: 10.1371/journal.pone.0194108. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

