Helicobacter pylori CagA EPIYA Motif Variations Affect Metabolic Activity in B Cells
- PMID: 34564597
- PMCID: PMC8473296
- DOI: 10.3390/toxins13090592
Helicobacter pylori CagA EPIYA Motif Variations Affect Metabolic Activity in B Cells
Abstract
Background: Helicobacter pylori (Hp) colonizes the human stomach and can induce gastric cancer and mucosa-associated lymphoid tissue (MALT) lymphoma. Clinical observations suggest a role for the Hp virulence factor cytotoxin-associated gene A (CagA) in pathogenesis. The pathogenic activity of CagA is partly regulated by tyrosine phosphorylation of C-terminal Glu-Pro-Ile-Tyr-Ala (EPIYA) motifs in host cells. However, CagA differs considerably in EPIYA motifs, whose functions have been well characterized in epithelial cells. Since CagA is fragmented in immune cells, different CagA variants may exhibit undetected functions in B cells.
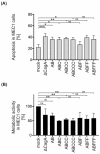
Methods: B cells were infected with Hp isolates and isogenic mutants expressing different CagA EPIYA variants. CagA translocation and tyrosine phosphorylation were investigated by Western blotting. Apoptosis was analyzed by flow cytometry and metabolic activity was detected by an MTT assay.
Results: Isogenic CagA EPIYA variants are equally well translocated into B cells, followed by tyrosine phosphorylation and cleavage. B cell apoptosis was induced in a CagA-independent manner. However, variants containing at least one EPIYA-C motif affected metabolic activity independently of phosphorylation or multiplication of EPIYA-C motifs.
Conclusions: The diverse structure of CagA regulates B cell physiology, whereas B cell survival is independent of CagA.
Keywords: B cells; CagA; EPIYA motifs; Helicobacter pylori.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hoy B., Löwer M., Weydig C., Carra G., Tegtmeyer N., Geppert T., Schröder P., Sewald N., Backert S., Schneider G., et al. Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Rep. 2010;11:798–804. doi: 10.1038/embor.2010.114. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

