Measurement over 1 Year of Neutralizing Antibodies in Cattle Immunized with Trivalent Vaccines Recombinant Alpha, Beta and Epsilon of Clostridium perfringens
- PMID: 34564599
- PMCID: PMC8470993
- DOI: 10.3390/toxins13090594
Measurement over 1 Year of Neutralizing Antibodies in Cattle Immunized with Trivalent Vaccines Recombinant Alpha, Beta and Epsilon of Clostridium perfringens
Abstract
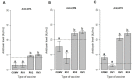
The alpha (CPA), beta (CPB) and epsilon (ETX) toxins of Clostridium perfringens are responsible for causing diseases that are difficult to eradicate and have lethal potential in production animals. Vaccination of herds is still the best control strategy. Recombinant clostridial vaccines have shown good success at inducing neutralizing antibody titers and appear to be a viable alternative to the conventional production of commercial clostridial toxoids. Research is still needed on the longevity of the humoral immune response induced by recombinant proteins in immunized animals, preferably in target species. The objective of this study was to measure the humoral immune response of cattle immunized with trivalent vaccines containing the recombinant proteins alpha (rCPA), beta (rCPB) and epsilon (rETX) of C. perfringens produced in Escherichia coli at three different concentrations (100, 200, and 400 µg) of each protein for 12 months. The recombinant vaccines containing 200 (RV2) and 400 µg (RV3) yielded statistically similar results at 56 days. They performed better throughout the study period because they induced higher neutralizing antibody titers and were detectable for up to 150 and 180 days, respectively. Regarding industrial-scale production, RV2 would be the most economical and viable formulation as it achieved results similar to RV3 at half the concentration of recombinant proteins in its formulation. However, none of the vaccines tested induced the production of detectable antibody titers on day 365 of the experiment, the time of revaccination typically recommended in vaccination protocols. Thus, reiterating the need for research in the field of vaccinology to achieve greater longevity of the humoral immune response against these clostridial toxins in animals, in addition to the need to discuss the vaccine schedules and protocols adopted in cattle production.
Keywords: antibody curve; biotechnology; immunology; recombinant alpha protein; recombinant beta protein; recombinant epsilon protein; serum neutralization; vaccine protocols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Immunogenicity of a Trivalent Recombinant Vaccine Against Clostridium perfringens Alpha, Beta, and Epsilon Toxins in Farm Ruminants.Sci Rep. 2016 Mar 23;6:22816. doi: 10.1038/srep22816. Sci Rep. 2016. PMID: 27004612 Free PMC article.
-
Potential protective immunogenicity of recombinant Clostridium perfringens α-β2-β1 fusion toxin in mice, sows and cows.Vaccine. 2011 Jul 26;29(33):5459-66. doi: 10.1016/j.vaccine.2011.05.059. Epub 2011 Jun 12. Vaccine. 2011. PMID: 21641956
-
Humoral Immune Response Evaluation in Horses Vaccinated with Recombinant Clostridium perfringens Toxoids Alpha and Beta for 12 Months.Toxins (Basel). 2021 Aug 13;13(8):566. doi: 10.3390/toxins13080566. Toxins (Basel). 2021. PMID: 34437437 Free PMC article.
-
Recombinant Alpha, Beta, and Epsilon Toxins of Clostridium perfringens: Production Strategies and Applications as Veterinary Vaccines.Toxins (Basel). 2016 Nov 21;8(11):340. doi: 10.3390/toxins8110340. Toxins (Basel). 2016. PMID: 27879630 Free PMC article. Review.
-
Vaccines against Clostridium perfringens alpha-toxin.Curr Pharm Biotechnol. 2013;14(10):913-7. doi: 10.2174/1389201014666131226124348. Curr Pharm Biotechnol. 2013. PMID: 24372250 Review.
Cited by
-
Clostridial Infections in Cattle: A Comprehensive Review with Emphasis on Current Data Gaps in Brazil.Animals (Basel). 2024 Oct 10;14(20):2919. doi: 10.3390/ani14202919. Animals (Basel). 2024. PMID: 39457848 Free PMC article. Review.
-
Sheep immune response against a novel recombinant enterotoxemia and infectious necrotic hepatitis vaccine in Türkiye.BMC Vet Res. 2025 May 28;21(1):380. doi: 10.1186/s12917-025-04841-6. BMC Vet Res. 2025. PMID: 40426194 Free PMC article.
-
Humoral and cellular immune responses in cattle upon Clostridium chauvoei vaccination and challenge.Front Immunol. 2025 May 20;16:1584168. doi: 10.3389/fimmu.2025.1584168. eCollection 2025. Front Immunol. 2025. PMID: 40463373 Free PMC article.
References
-
- ABIEC—Associação Brasileira Das Indústrias Exportadoras de Carnes. [(accessed on 15 April 2020)]. Available online: http://abiec.com.br/
-
- Moreira G.M.S.G., Salvarani F.M., da Cunha C.E.P., Mendonça M., Moreira Â.N., Gonçalves L.A., Pires P.S., Lobato F.C.F., Conceição F.R. Immunogenicity of a Trivalent Recombinant Vaccine Against Clostridium perfringens Alpha, Beta, and Epsilon Toxins in Farm Ruminants. Sci. Rep. 2016;6:22816. doi: 10.1038/srep22816. - DOI - PMC - PubMed
-
- Silva R.O.S., Duarte M.C., Oliveira Junior C.A., de Assis R.A., Lana A.M.Q., Lobato F.C.F. Comparison of Humoral Neutralizing Antibody Response in Rabbits, Guinea Pigs, and Cattle Vaccinated with Epsilon and Beta Toxoids from Clostridium perfringens and C. Botulinum Types C and D Toxoids. Anaerobe. 2018;54:19–22. doi: 10.1016/j.anaerobe.2018.07.014. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

