Force Mapping Study of Actinoporin Effect in Membranes Presenting Phase Domains
- PMID: 34564674
- PMCID: PMC8473010
- DOI: 10.3390/toxins13090669
Force Mapping Study of Actinoporin Effect in Membranes Presenting Phase Domains
Abstract
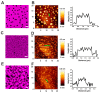
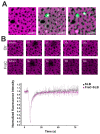
Equinatoxin II (EqtII) and Fragaceatoxin C (FraC) are pore-forming toxins (PFTs) from the actinoporin family that have enhanced membrane affinity in the presence of sphingomyelin (SM) and phase coexistence in the membrane. However, little is known about the effect of these proteins on the nanoscopic properties of membrane domains. Here, we used combined confocal microscopy and force mapping by atomic force microscopy to study the effect of EqtII and FraC on the organization of phase-separated phosphatidylcholine/SM/cholesterol membranes. To this aim, we developed a fast, high-throughput processing tool to correlate structural and nano-mechanical information from force mapping. We found that both proteins changed the lipid domain shape. Strikingly, they induced a reduction in the domain area and circularity, suggesting a decrease in the line tension due to a lipid phase height mismatch, which correlated with proteins binding to the domain interfaces. Moreover, force mapping suggested that the proteins affected the mechanical properties at the edge, but not in the bulk, of the domains. This effect could not be revealed by ensemble force spectroscopy measurements supporting the suitability of force mapping to study local membrane topographical and mechanical alterations by membranotropic proteins.
Keywords: atomic force microscopy; force spectroscopy; membrane phase domains; pore forming toxins.
Conflict of interest statement
The authors declare no conflict of interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

