Seroconversion After Coronavirus Disease 2019 Vaccination in Patients Awaiting Liver Transplantation: Fact or Fancy?
- PMID: 34564945
- PMCID: PMC8662269
- DOI: 10.1002/lt.26312
Seroconversion After Coronavirus Disease 2019 Vaccination in Patients Awaiting Liver Transplantation: Fact or Fancy?
Abstract
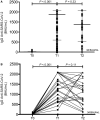
Chronic liver disease increased the risk of severe coronavirus disease 2019 (COVID-19). Trials to assess efficacy/safety of COVID-19 vaccines in liver disease are underway. We evaluated the humoral immune response and safety of anti-COVID-19 vaccination among patients waiting liver transplantation (LT). We enrolled all pre-LT adults who completed anti-COVID-19 vaccination between January 2021-August 2021 as study group. Patients with histories of COVID-19 received 1 vaccine dose, and all others received 2 doses. Patients were tested for COVID-19 immunoglobulin G (IgG) within 1 and 2 months after vaccination. Safety was evaluated with telephone interviews/outpatient visits. A control group of 30 healthcare workers who underwent vaccination in January 2021 and tested for IgG after 4 months was included. In the 89 pre-LT patients, at T1 (23 days after vaccination), seroconversion rate was 94.4%, and median IgG value was 1980 binding antibody units/mL (interquartile range 646-2080), and at T2 (68 days after vaccination) was 92.0%, with IgG value of 1450 (577-2080); (T1 versus T2, P = 0.38). In the 10/89 patients who received 1 vaccine dose, the median IgG value was 274 (68-548) before vaccine (T0), 2080 (1165-2080) at T1, and 2030 (964-2080) at T2 (T0 versus T1, P = 0.03; T1 versus T2, P = 0.99). All controls tested positive at 4 months after vaccination, with a median value of 847 (509-1165; P < 0.001 versus T1 and P = 0.04 versus T2 in the study group). No serious adverse event was reported in both groups. Our data from 89 pre-LT patients suggest a high rate of immunization (94.4%) after a median time of 23 days from safe COVID-19 vaccine. None of the patients developed COVID-19.
Copyright © 2021 American Association for the Study of Liver Diseases.
Figures

Comment in
-
Seroconversion After Coronavirus Disease 2019 Vaccination.Liver Transpl. 2022 Mar;28(3):514. doi: 10.1002/lt.26327. Epub 2021 Nov 25. Liver Transpl. 2022. PMID: 34619023 Free PMC article. No abstract available.

