Effects of Lifestyle Modification on Patients With Resistant Hypertension: Results of the TRIUMPH Randomized Clinical Trial
- PMID: 34565172
- PMCID: PMC8511053
- DOI: 10.1161/CIRCULATIONAHA.121.055329
Effects of Lifestyle Modification on Patients With Resistant Hypertension: Results of the TRIUMPH Randomized Clinical Trial
Abstract
Background: Although lifestyle modifications generally are effective in lowering blood pressure (BP) among patients with unmedicated hypertension and in those treated with 1 or 2 antihypertensive agents, the value of exercise and diet for lowering BP in patients with resistant hypertension is unknown.
Methods: One hundred forty patients with resistant hypertension (mean age, 63 years; 48% female; 59% Black; 31% with diabetes; 21% with chronic kidney disease) were randomly assigned to a 4-month program of lifestyle modification (C-LIFE [Center-Based Lifestyle Intervention]) including dietary counseling, behavioral weight management, and exercise, or a single counseling session providing SEPA (Standardized Education and Physician Advice). The primary end point was clinic systolic BP; secondary end points included 24-hour ambulatory BP and select cardiovascular disease biomarkers including baroreflex sensitivity to quantify the influence of the baroreflex on heart rate, high-frequency heart rate variability to assess vagally mediated modulation of heart rate, flow-mediated dilation to evaluate endothelial function, pulse wave velocity to assess arterial stiffness, and left ventricular mass to characterize left ventricular structure.
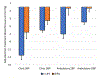
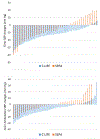
Results: Between-group comparisons revealed that the reduction in clinic systolic BP was greater in C-LIFE (-12.5 [95% CI, -14.9 to -10.2] mm Hg) compared with SEPA(-7.1 [-95% CI, 10.4 to -3.7] mm Hg) (P=0.005); 24-hour ambulatory systolic BP also was reduced in C-LIFE (-7.0 [95% CI, -8.5 to -4.0] mm Hg), with no change in SEPA (-0.3 [95% CI, -4.0 to 3.4] mm Hg) (P=0.001). Compared with SEPA, C-LIFE resulted in greater improvements in resting baroreflex sensitivity (2.3 ms/mm Hg [95% CI, 1.3 to 3.3] versus -1.1 ms/mm Hg [95% CI, -2.5 to 0.3]; P<0.001), high-frequency heart rate variability (0.4 ln ms2 [95% CI, 0.2 to 0.6] versus -0.2 ln ms2 [95% CI, -0.5 to 0.1]; P<0.001), and flow-mediated dilation (0.3% [95% CI, -0.3 to 1.0] versus -1.4% [95% CI, -2.5 to -0.3]; P=0.022). There were no between-group differences in pulse wave velocity (P=0.958) or left ventricular mass (P=0.596).
Conclusions: Diet and exercise can lower BP in patients with resistant hypertension. A 4-month structured program of diet and exercise as adjunctive therapy delivered in a cardiac rehabilitation setting results in significant reductions in clinic and ambulatory BP and improvement in selected cardiovascular disease biomarkers. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT02342808.
Keywords: DASH diet; cardiac rehabilitation; exercise; hypertension; lifestyle.
Conflict of interest statement
Figures

References
-
- Noubiap JJ, Nansseu JR, Nyaga UF, Sime PS, Francis I and Bigna JJ. Global prevalence of resistant hypertension: a meta-analysis of data from 3.2 million patients. Heart. 2019;105:98–105. - PubMed
-
- Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. - PubMed
-
- Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–2068. - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

