Plasma Kallikrein as a Modulator of Liver Injury/Remodeling
- PMID: 34566641
- PMCID: PMC8458624
- DOI: 10.3389/fphar.2021.715111
Plasma Kallikrein as a Modulator of Liver Injury/Remodeling
Abstract
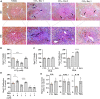
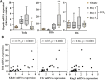
The occurrence and persistence of hepatic injury which arises from cell death and inflammation result in liver disease. The processes that lead to liver injury progression and resolution are still not fully delineated. The plasma kallikrein-kinin system (PKKS) has been shown to play diverse functions in coagulation, tissue injury, and inflammation, but its role in liver injury has not been defined yet. In this study, we have characterized the role of the PKKS at various stages of liver injury in mice, as well as the direct effects of plasma kallikrein on human hepatocellular carcinoma cell line (HepG2). Histological, immunohistochemical, and gene expression analyses were utilized to assess cell injury on inflammatory and fibrotic factors. Acute liver injury triggered by carbon tetrachloride (CCl4) injection resulted in significant upregulation of the plasma kallikrein gene (Klkb1) and was highly associated with the high mobility group box 1 gene, the marker of cell death (r = 0.75, p < 0.0005, n = 7). In addition, increased protein expression of plasma kallikrein was observed as clusters around necrotic areas. Plasma kallikrein treatment significantly increased the proliferation of CCl4-induced HepG2 cells and induced a significant increase in the gene expression of the thrombin receptor (protease activated receptor-1), interleukin 1 beta, and lectin-galactose binding soluble 3 (galectin-3) (p < 0.05, n = 4). Temporal variations in the stages of liver fibrosis were associated with an increase in the mRNA levels of bradykinin receptors: beta 1 and 2 genes (p < 0.05; n = 3-10). In conclusion, these findings indicate that plasma kallikrein may play diverse roles in liver injury, inflammation, and fibrosis, and suggest that plasma kallikrein may be a target for intervention in the states of liver injury.
Keywords: fibrosis; inflammation; kallikrein-kinin system; liver injury; necrosis; plasma kallikrein; remodeling.
Copyright © 2021 Ahmed, Jaffa, Moussa, Hatem, El-Achkar, Al Sayegh, Karam, Hamade, Habib and Jaffa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Abdallah R. T., Keum J.-S., Lee M.-H., Wang B., Gooz M., Luttrell D. K., et al. (2010). Plasma Kallikrein Promotes Epidermal Growth Factor Receptor Transactivation and Signaling in Vascular Smooth Muscle through Direct Activation of Protease-Activated Receptors. J. Biol. Chem. 285 (45), 35206–35215. 10.1074/jbc.M110.171769 - DOI - PMC - PubMed
-
- Borensztajn K., Stiekema J., Nijmeijer S., Reitsma P. H., Peppelenbosch M. P., Spek C. A. (2008). Factor Xa Stimulates Proinflammatory and Profibrotic Responses in Fibroblasts via Protease-Activated Receptor-2 Activation. Am. J. Pathol. 172 (2), 309–320. 10.2353/ajpath.2008.070347 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

