Effects of High-Fat and High-Fat/High-Sucrose Diet-Induced Obesity on PVAT Modulation of Vascular Function in Male and Female Mice
- PMID: 34566644
- PMCID: PMC8460896
- DOI: 10.3389/fphar.2021.720224
Effects of High-Fat and High-Fat/High-Sucrose Diet-Induced Obesity on PVAT Modulation of Vascular Function in Male and Female Mice
Abstract
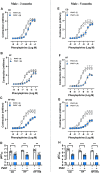
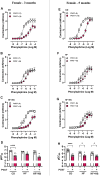
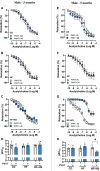
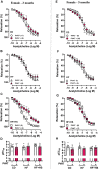
Increased adiposity in perivascular adipose tissue (PVAT) has been related to vascular dysfunction. High-fat (HF) diet-induced obesity models are often used to analyze the translational impact of obesity, but differences in sex and Western diet type complicate comparisons between studies. The role of PVAT was investigated in small mesenteric arteries (SMAs) of male and female mice fed a HF or a HF plus high-sucrose (HF + HS) diet for 3 or 5 months and compared them to age/sex-matched mice fed a chow diet. Vascular responses of SMAs without (PVAT-) or with PVAT (PVAT+) were evaluated. HF and HF + HS diets increased body weight, adiposity, and fasting glucose and insulin levels without affecting blood pressure and circulating adiponectin levels in both sexes. HF or HF + HS diet impaired PVAT anticontractile effects in SMAs from females but not males. PVAT-mediated endothelial dysfunction in SMAs from female mice after 3 months of a HF + HS diet, whereas in males, this effect was observed only after 5 months of HF + HS diet. However, PVAT did not impact acetylcholine-induced relaxation in SMAs from both sexes fed HF diet. The findings suggest that the addition of sucrose to a HF diet accelerates PVAT dysfunction in both sexes. PVAT dysfunction in response to both diets was observed early in females compared to age-matched males suggesting a susceptibility of the female sex to PVAT-mediated vascular complications in the setting of obesity. The data illustrate the importance of the duration and composition of obesogenic diets for investigating sex-specific treatments and pharmacological targets for obesity-induced vascular complications.
Keywords: endothelial dysfunction; obesity; perivascular adipose tissue; resistance arteries; sex differences.
Copyright © 2021 Victorio, Guizoni, Freitas, Araujo and Davel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abarca-Gómez L., Abdeen Z. A., Hamid Z. A., Abu-Rmeileh N. M., Acosta-Cazares B., Acuin C. E. A. (2017). Worldwide Trends in Body-Mass index, Underweight, Overweight, and Obesity from 1975 to 2016: a Pooled Analysis of 2416 Population-Based Measurement Studies in 128·9 Million Children, Adolescents, and Adults. Lancet 390, 2627–2642. 10.1016/S0140-6736(17)32129-3 - DOI - PMC - PubMed
-
- Barris C., Faulkner J., de Chantemele E. B. (2021). Loss of Female Sex Hormones Does Not Induce a Mechanistic Switch for Development of Hypertension in Obese Female Mice - Barris - 2021. Experimental Biology 2021 Meeting abstracts. 35(S1), 10.1096/fasebj.2021.35.S1.04280 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

