Polysaccharides Extracted From Panax Ginseng C.A. Mey Enhance Complement Component 4 Biosynthesis in Human Hepatocytes
- PMID: 34566655
- PMCID: PMC8461058
- DOI: 10.3389/fphar.2021.734394
Polysaccharides Extracted From Panax Ginseng C.A. Mey Enhance Complement Component 4 Biosynthesis in Human Hepatocytes
Abstract
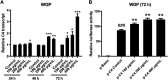
Panax ginseng C.A. Mey (ginseng) is a classic medicinal plant which is well known for enhancing immune capacity. Polysaccharides are one of the main active components of ginseng. We isolated water-soluble ginseng polysaccharides (WGP) and analyzed the physicochemical properties of WGP including molecular weight, monosaccharide composition, and structural characteristics. WGP had minimal effect on the growth of hepatocytes. Interestingly, WGP significantly increased the mRNA and protein levels of complement component 4 (C4), one of the core components of the complement system. Promoter reporter gene assays revealed that WGP significantly enhanced activity of the C4 gene promoter. Deletion analyses determined that the E-box1 and Sp1 regions play key roles in WGP-induced C4 transcription. Taken together, our results suggest that WGP promotes C4 biosynthesis through upregulation of transcription. These results provide new explanation for the intrinsic mechanism by which ginseng boosts human immune capacity.
Keywords: C4 promoter; C4 transcription; complement component 4; ginseng; water-soluble ginseng polysaccharides.
Copyright © 2021 Liu, Liu, Wang, Liu, Hu, Sun and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

