The Novel Antioxidant Compound JSH-23 Prevents Osteolysis by Scavenging ROS During Both Osteoclastogenesis and Osteoblastogenesis
- PMID: 34566656
- PMCID: PMC8458573
- DOI: 10.3389/fphar.2021.734774
The Novel Antioxidant Compound JSH-23 Prevents Osteolysis by Scavenging ROS During Both Osteoclastogenesis and Osteoblastogenesis
Abstract
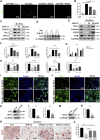
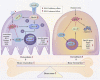
Inflammatory osteolysis is a pathological skeletal disease associated with not only the production of inflammatory cytokines but also local oxidative status. Excessive reactive oxygen species (ROS) promote bone resorption by osteoclasts and induce the apoptosis of osteoblasts. In consideration of the lack of effective preventive or treatments options against osteolysis, the exploitation of novel pharmacological compounds/agents is critically required. In our study, we found that a novel antioxidant compound, JSH-23, plays a role in restoring bone homeostasis by scavenging intracellular ROS during both osteoclastogenesis and osteoblastogenesis. Mechanically, JSH-23 suppressed RANKL-induced osteoclastogenesis, bone resorption and the expression of specific genes (including NFATc1, c-Fos, TRAP, CTSK and DC-STAMP) via inhibition of the NF-κB signaling pathway. Meanwhile, JSH-23 suppressed RANKL-induced ROS generation via the TRAF6/Rac1/NOX1 pathway and the enhanced expression of Nrf2/HO-1. In addition, JSH-23 attenuated H2O2-induced apoptosis and mineralization reduction in osteoblasts by reducing ROS production and enhancing Nrf2/HO-1 expression. Our in vivo results further revealed that JSH-23 exerts its protective effects on bone mass through its antioxidant activity. In conclusion, our results show that the application of JSH-23 might be a novel and plausible strategy for the treatment of osteolysis-related disease.
Keywords: HO-1 (heme oxygenase-1); JSH-23 (PubChem CID: 16760588); Nrf2; ROS–reactive oxygen species; osteoblast (OB); osteoclast (OC).
Copyright © 2021 Mei, Zheng, Ma, Xia, Gao, Hao, Luo and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Arias-Salvatierra D., Silbergeld E. K., Acosta-Saavedra L. C., Calderon-Aranda E. S. (2011). Role of Nitric Oxide Produced by iNOS through NF-κB Pathway in Migration of Cerebellar Granule Neurons Induced by Lipopolysaccharide. Cell Signal 23 (2), 425–435. 10.1016/j.cellsig.2010.10.017 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

