Liver Zonation - Revisiting Old Questions With New Technologies
- PMID: 34566696
- PMCID: PMC8458816
- DOI: 10.3389/fphys.2021.732929
Liver Zonation - Revisiting Old Questions With New Technologies
Abstract
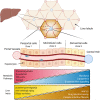
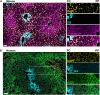
Despite the ever-increasing prevalence of non-alcoholic fatty liver disease (NAFLD), the etiology and pathogenesis remain poorly understood. This is due, in part, to the liver's complex physiology and architecture. The liver maintains glucose and lipid homeostasis by coordinating numerous metabolic processes with great efficiency. This is made possible by the spatial compartmentalization of metabolic pathways a phenomenon known as liver zonation. Despite the importance of zonation to normal liver function, it is unresolved if and how perturbations to liver zonation can drive hepatic pathophysiology and NAFLD development. While hepatocyte heterogeneity has been identified over a century ago, its examination had been severely hindered due to technological limitations. Recent advances in single cell analysis and imaging technologies now permit further characterization of cells across the liver lobule. This review summarizes the advances in examining liver zonation and elucidating its regulatory role in liver physiology and pathology. Understanding the spatial organization of metabolism is vital to further our knowledge of liver disease and to provide targeted therapeutic avenues.
Keywords: architecture; liver; physiology; technologies; zonation.
Copyright © 2021 Cunningham and Porat-Shliom.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

