The Mechanobiology of Endothelial-to-Mesenchymal Transition in Cardiovascular Disease
- PMID: 34566697
- PMCID: PMC8458763
- DOI: 10.3389/fphys.2021.734215
The Mechanobiology of Endothelial-to-Mesenchymal Transition in Cardiovascular Disease
Abstract
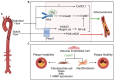
Endothelial cells (ECs) lining the cardiovascular system are subjected to a highly dynamic microenvironment resulting from pulsatile pressure and circulating blood flow. Endothelial cells are remarkably sensitive to these forces, which are transduced to activate signaling pathways to maintain endothelial homeostasis and respond to changes in the environment. Aberrations in these biomechanical stresses, however, can trigger changes in endothelial cell phenotype and function. One process involved in this cellular plasticity is endothelial-to-mesenchymal transition (EndMT). As a result of EndMT, ECs lose cell-cell adhesion, alter their cytoskeletal organization, and gain increased migratory and invasive capabilities. EndMT has long been known to occur during cardiovascular development, but there is now a growing body of evidence also implicating it in many cardiovascular diseases (CVD), often associated with alterations in the cellular mechanical environment. In this review, we highlight the emerging role of shear stress, cyclic strain, matrix stiffness, and composition associated with EndMT in CVD. We first provide an overview of EndMT and context for how ECs sense, transduce, and respond to certain mechanical stimuli. We then describe the biomechanical features of EndMT and the role of mechanically driven EndMT in CVD. Finally, we indicate areas of open investigation to further elucidate the complexity of EndMT in the cardiovascular system. Understanding the mechanistic underpinnings of the mechanobiology of EndMT in CVD can provide insight into new opportunities for identification of novel diagnostic markers and therapeutic interventions.
Keywords: biomechanical; cardiovascular disease; endothelial; endothelial-to-mesenchymal transition; mechanobiology; mesenchymal.
Copyright © 2021 Islam, Boström, Di Carlo, Simmons, Tintut, Yao and Hsu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

