Age-at-Injury Determines the Extent of Long-Term Neuropathology and Microgliosis After a Diffuse Brain Injury in Male Rats
- PMID: 34566867
- PMCID: PMC8455817
- DOI: 10.3389/fneur.2021.722526
Age-at-Injury Determines the Extent of Long-Term Neuropathology and Microgliosis After a Diffuse Brain Injury in Male Rats
Abstract
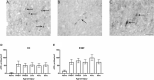
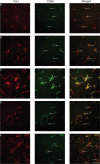
Traumatic brain injury (TBI) can occur at any age, from youth to the elderly, and its contribution to age-related neuropathology remains unknown. Few studies have investigated the relationship between age-at-injury and pathophysiology at a discrete biological age. In this study, we report the immunohistochemical analysis of naïve rat brains compared to those subjected to diffuse TBI by midline fluid percussion injury (mFPI) at post-natal day (PND) 17, PND35, 2-, 4-, or 6-months of age. All brains were collected when rats were 10-months of age (n = 6-7/group). Generalized linear mixed models were fitted to analyze binomial proportion and count data with R Studio. Amyloid precursor protein (APP) and neurofilament (SMI34, SMI32) neuronal pathology were counted in the corpus callosum (CC) and primary sensory barrel field (S1BF). Phosphorylated TAR DNA-binding protein 43 (pTDP-43) neuropathology was counted in the S1BF and hippocampus. There was a significantly greater extent of APP and SMI34 axonal pathology and pTDP-43 neuropathology following a TBI compared with naïves regardless of brain region or age-at-injury. However, age-at-injury did determine the extent of dendritic neurofilament (SMI32) pathology in the CC and S1BF where all brain-injured rats exhibited a greater extent of pathology compared with naïve. No significant differences were detected in the extent of astrocyte activation between brain-injured and naïve rats. Microglia counts were conducted in the S1BF, hippocampus, ventral posteromedial (VPM) nucleus, zona incerta, and posterior hypothalamic nucleus. There was a significantly greater proportion of deramified microglia, regardless of whether the TBI was recent or remote, but this only occurred in the S1BF and hippocampus. The proportion of microglia with colocalized CD68 and TREM2 in the S1BF was greater in all brain-injured rats compared with naïve, regardless of whether the TBI was recent or remote. Only rats with recent TBI exhibited a greater proportion of CD68-positive microglia compared with naive in the hippocampus and posterior hypothalamic nucleus. Whilst, only rats with a remote brain-injury displayed a greater proportion of microglia colocalized with TREM2 in the hippocampus. Thus, chronic alterations in neuronal and microglial characteristics are evident in the injured brain despite the recency of a diffuse brain injury.
Keywords: TBI; age-at-injury; aging; concussion; juvenile; pathology; puberty; traumatic brain injury.
Copyright © 2021 Doust, Rowe, Adelson, Lifshitz and Ziebell.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








Similar articles
-
Age-At-Injury Influences the Glial Response to Traumatic Brain Injury in the Cortex of Male Juvenile Rats.Front Neurol. 2022 Jan 17;12:804139. doi: 10.3389/fneur.2021.804139. eCollection 2021. Front Neurol. 2022. PMID: 35111130 Free PMC article.
-
Aging with TBI vs. Aging: 6-month temporal profiles for neuropathology and astrocyte activation converge in behaviorally relevant thalamocortical circuitry of male and female rats.bioRxiv [Preprint]. 2023 Feb 7:2023.02.06.527058. doi: 10.1101/2023.02.06.527058. bioRxiv. 2023. PMID: 36798182 Free PMC article. Preprint.
-
Morphological and genetic activation of microglia after diffuse traumatic brain injury in the rat.Neuroscience. 2012 Dec 6;225:65-75. doi: 10.1016/j.neuroscience.2012.08.058. Epub 2012 Sep 6. Neuroscience. 2012. PMID: 22960311 Free PMC article.
-
Neuropathology in sensory, but not motor, brainstem nuclei of the rat whisker circuit after diffuse brain injury.Somatosens Mot Res. 2014 Sep;31(3):127-35. doi: 10.3109/08990220.2014.897602. Epub 2014 Apr 7. Somatosens Mot Res. 2014. PMID: 24702476
-
Traumatic brain injury (TBI) in collision sports: Possible mechanisms of transformation into chronic traumatic encephalopathy (CTE).Metabolism. 2019 Nov;100S:153943. doi: 10.1016/j.metabol.2019.07.007. Metabolism. 2019. PMID: 31610856 Review.
Cited by
-
Amelioration of Brain Damage after Treatment with the Methanolic Extract of Glycyrrhizae Radix et Rhizoma in Mice.Pharmaceutics. 2022 Dec 12;14(12):2776. doi: 10.3390/pharmaceutics14122776. Pharmaceutics. 2022. PMID: 36559268 Free PMC article.
-
Age-At-Injury Influences the Glial Response to Traumatic Brain Injury in the Cortex of Male Juvenile Rats.Front Neurol. 2022 Jan 17;12:804139. doi: 10.3389/fneur.2021.804139. eCollection 2021. Front Neurol. 2022. PMID: 35111130 Free PMC article.
-
Drosophila melanogaster as a model to study age and sex differences in brain injury and neurodegeneration after mild head trauma.Front Neurosci. 2023 Apr 3;17:1150694. doi: 10.3389/fnins.2023.1150694. eCollection 2023. Front Neurosci. 2023. PMID: 37077318 Free PMC article. Review.
-
Temporal changes in the microglial proteome of male and female mice after a diffuse brain injury using label-free quantitative proteomics.Glia. 2023 Apr;71(4):880-903. doi: 10.1002/glia.24313. Epub 2022 Dec 5. Glia. 2023. PMID: 36468604 Free PMC article.
-
Methanol extract of Ligusticum chuanxiong Hort. Rhizome ameliorates bilateral common carotid artery stenosis-induced cognitive deficit in mice by altering microglia and astrocyte activation.Front Pharmacol. 2024 Mar 14;15:1329895. doi: 10.3389/fphar.2024.1329895. eCollection 2024. Front Pharmacol. 2024. PMID: 38549667 Free PMC article.
References
LinkOut - more resources
Full Text Sources

