Association of Polygenetic Risk Scores Related to Immunity and Inflammation with Hyperthyroidism Risk and Interactions between the Polygenetic Scores and Dietary Factors in a Large Cohort
- PMID: 34567510
- PMCID: PMC8457978
- DOI: 10.1155/2021/7664641
Association of Polygenetic Risk Scores Related to Immunity and Inflammation with Hyperthyroidism Risk and Interactions between the Polygenetic Scores and Dietary Factors in a Large Cohort
Abstract
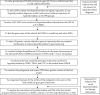
Graves's disease and thyroiditis induce hyperthyroidism, the causes of which remain unclear, although they are involved with genetic and environmental factors. We aimed to evaluate polygenetic variants for hyperthyroidism risk and their interaction with metabolic parameters and nutritional intakes in an urban hospital-based cohort. A genome-wide association study (GWAS) of participants with (cases; n = 842) and without (controls, n = 38,799) hyperthyroidism was used to identify and select genetic variants. In clinical and lifestyle interaction with PRS, 312 participants cured of hyperthyroidism were excluded. Single nucleotide polymorphisms (SNPs) associated with gene-gene interactions were selected by hyperthyroidism generalized multifactor dimensionality reduction. Polygenic risk scores (PRSs) were generated by summing the numbers of selected SNP risk alleles. The best gene-gene interaction model included tumor-necrosis factor (TNF)_rs1800610, mucin 22 (MUC22)_rs1304322089, tribbles pseudokinase 2 (TRIB2)_rs1881145, cytotoxic T-lymphocyte-associated antigen 4 (CTLA4)_rs231775, lipoma-preferred partner (LPP)_rs6780858, and human leukocyte antigen (HLA)-J_ rs767861647. The PRS of the best model was positively associated with hyperthyroidism risk by 1.939-fold (1.317-2.854) after adjusting for covariates. PRSs interacted with age, metabolic syndrome, and dietary inflammatory index (DII), while hyperthyroidism risk interacted with energy, calcium, seaweed, milk, and coffee intake (P < 0.05). The PRS impact on hyperthyroidism risk was observed in younger (<55 years) participants and adults without metabolic syndrome. PRSs were positively associated with hyperthyroidism risk in participants with low dietary intakes of energy (OR = 2.74), calcium (OR = 2.84), seaweed (OR = 3.43), milk (OR = 2.91), coffee (OR = 2.44), and DII (OR = 3.45). In conclusion, adults with high PRS involved in inflammation and immunity had a high hyperthyroidism risk exacerbated under low intakes of energy, calcium, seaweed, milk, or coffee. These results can be applied to personalized nutrition in a clinical setting.
Copyright © 2021 Mi Young Song and Sunmin Park.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Association between Polygenetic Risk Scores of Low Immunity and Interactions between These Scores and Moderate Fat Intake in a Large Cohort.Nutrients. 2021 Aug 19;13(8):2849. doi: 10.3390/nu13082849. Nutrients. 2021. PMID: 34445011 Free PMC article.
-
Association of Polygenetic Risk Scores Related to Cell Differentiation and Inflammation with Thyroid Cancer Risk and Genetic Interaction with Dietary Intake.Cancers (Basel). 2021 Mar 25;13(7):1510. doi: 10.3390/cancers13071510. Cancers (Basel). 2021. PMID: 33805984 Free PMC article.
-
Association between polygenetic risk scores related to sarcopenia risk and their interactions with regular exercise in a large cohort of Korean adults.Clin Nutr. 2021 Oct;40(10):5355-5364. doi: 10.1016/j.clnu.2021.09.003. Epub 2021 Sep 9. Clin Nutr. 2021. PMID: 34560606
-
Statistical genetics and polygenic risk score for precision medicine.Inflamm Regen. 2021 Jun 17;41(1):18. doi: 10.1186/s41232-021-00172-9. Inflamm Regen. 2021. PMID: 34140035 Free PMC article. Review.
-
The emerging field of polygenic risk scores and perspective for use in clinical care.Hum Mol Genet. 2020 Oct 20;29(R2):R165-R176. doi: 10.1093/hmg/ddaa136. Hum Mol Genet. 2020. PMID: 32620971 Review.
Cited by
-
Interactions between Polygenetic Variants and Lifestyle Factors in Hypothyroidism: A Hospital-Based Cohort Study.Nutrients. 2023 Sep 3;15(17):3850. doi: 10.3390/nu15173850. Nutrients. 2023. PMID: 37686882 Free PMC article.
-
Identification of potential immunotherapeutic targets and prognostic biomarkers in Graves' disease using weighted gene co-expression network analysis.Heliyon. 2024 Feb 28;10(5):e27175. doi: 10.1016/j.heliyon.2024.e27175. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38468967 Free PMC article.
-
Diet Quality and Dietary Inflammatory Index in Dutch Inflammatory Bowel Disease and Irritable Bowel Syndrome Patients.Nutrients. 2022 May 6;14(9):1945. doi: 10.3390/nu14091945. Nutrients. 2022. PMID: 35565912 Free PMC article.
-
Genome-wide association studies of coffee intake in UK/US participants of European ancestry uncover cohort-specific genetic associations.Neuropsychopharmacology. 2024 Sep;49(10):1609-1618. doi: 10.1038/s41386-024-01870-x. Epub 2024 Jun 11. Neuropsychopharmacology. 2024. PMID: 38858598
References
-
- Han X. R., Wen X., Wang S., et al. Correlations of CTLA‐4 exon‐1 49 A/G and promoter region 318C/T polymorphisms with the therapeutic efficacy of 131 I radionuclide in Graves’ disease in Chinese Han population. Journal of Cellular Biochemistry. 2018;119(8):6383–6390. doi: 10.1002/jcb.26327. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

